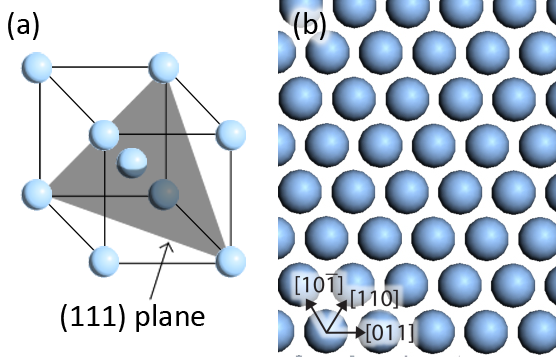
The crystal structure of silver is body-centred cubic (Figure 4.4a), and so the Ag(111) plane exhibits a close-packed, hexagonal structure (Figure 4.4b). Ag is a stable transition metal, and one of the few metals found abundantly in nature as a pure native element. This stability is due to its relative inertness, and the Ag(111) close-packed surface has the lowest energy of its high-symmetry surfaces.

As discussed in Section 4.2, molecules on Ag(111) tend to favour close-packed arrangements where possible. Its low surface energy allows molecules to easily diffuse until they encounter either a step edge (with a higher energy due to symmetry breaking), or another molecule (with whom the intermolecular interaction is stronger than the molecule–substrate interaction), giving rise to close-packed monolayers. The surface is not completely ignored by the deposited molecules however, with the choice of substrate playing a major role in the properties of NiDPP and MnClTPP (Chapter 5).
Ag/Si(111)-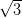 ×
×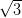 R30° consists of the honeycomb-chain trimer
structure. Cleaving the (111) surface of Si creates dangling bonds that point
out of the surface into the vacuum. If it is not passivated, these bonds
reconstruct into chains of dimers, however when Ag is deposited onto the
Si(111) surface, it forms pseudo-hexagons on the surface, which cause
the Si dangling bonds to reconstruct into trimers [187–189], as shown in
Figure 4.5.
R30° consists of the honeycomb-chain trimer
structure. Cleaving the (111) surface of Si creates dangling bonds that point
out of the surface into the vacuum. If it is not passivated, these bonds
reconstruct into chains of dimers, however when Ag is deposited onto the
Si(111) surface, it forms pseudo-hexagons on the surface, which cause
the Si dangling bonds to reconstruct into trimers [187–189], as shown in
Figure 4.5.
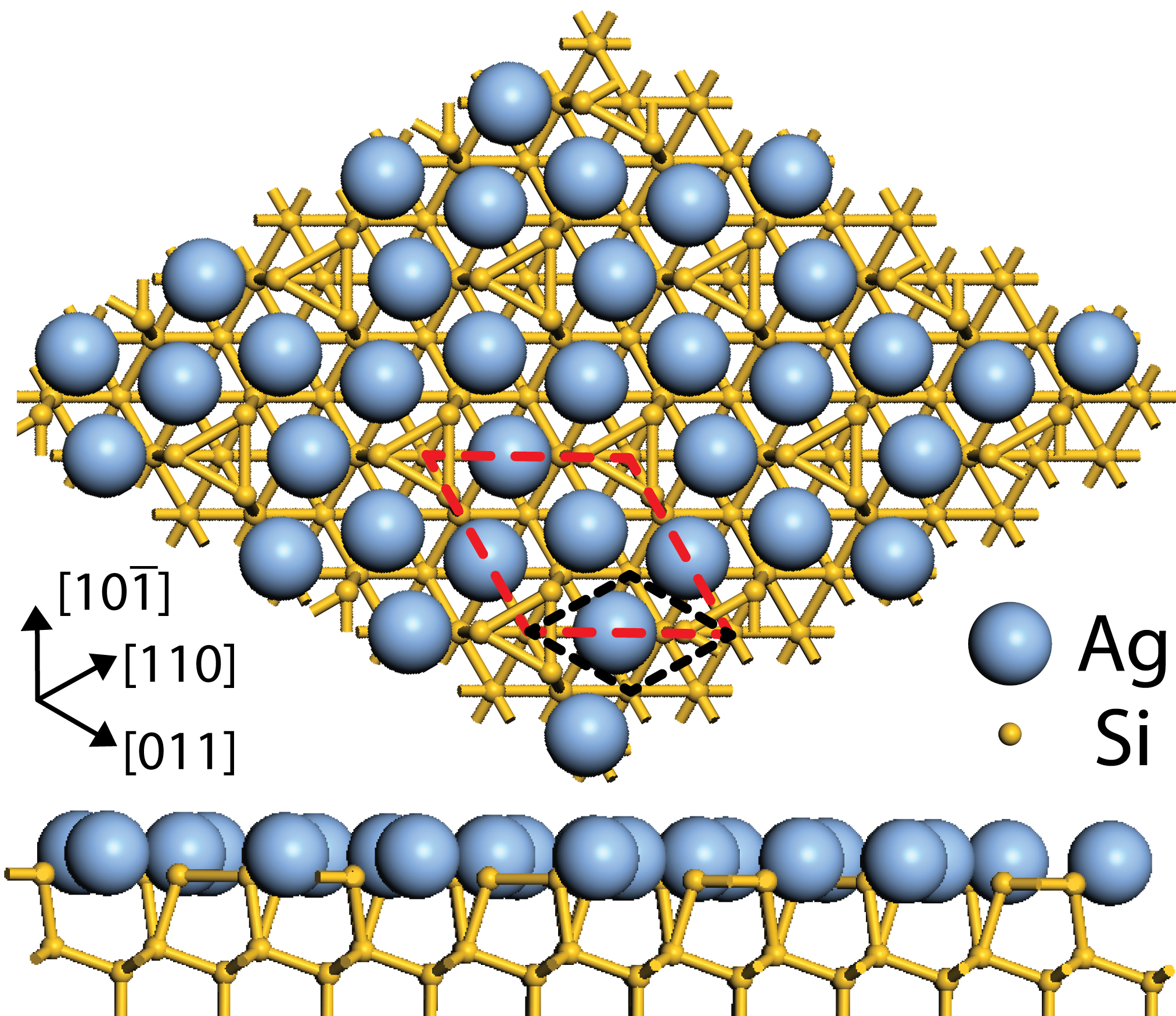
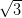 ×
×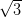 R30°
surface. Ag atoms are represented by large blue spheres, and Si as small
yellow spheres. When Ag is deposited on the Si(111) surface, it reconstructs
to form the honeycomb-chain-trimer structure [189]. The unit cell of Si(111)
is shown in black, with the
R30°
surface. Ag atoms are represented by large blue spheres, and Si as small
yellow spheres. When Ag is deposited on the Si(111) surface, it reconstructs
to form the honeycomb-chain-trimer structure [189]. The unit cell of Si(111)
is shown in black, with the 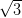 ×
×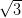 R30° reconstruction shown in red.
R30° reconstruction shown in red.
The Ag/Si(111)-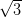 ×
×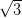 R30° surface was chosen as its reactivity is
expected to be intermediate in strength between that of clean Si(111) and
hydrogen-passivated Si(111) [190, 191]. On clean Si(111), porphyrin molecules
form covalent bonds and are unable to diffuse or self-assemble into ordered
structures at room temperature [161, 181], while on the H-passivated surface
they diffuse freely and can form islands [181, 192]. The effect of the Ag(111)
surface on the NiDPP self-assembly is expected to be minor, similar to that of
H-passivated Si(111), however the Ag/Si(111)-
R30° surface was chosen as its reactivity is
expected to be intermediate in strength between that of clean Si(111) and
hydrogen-passivated Si(111) [190, 191]. On clean Si(111), porphyrin molecules
form covalent bonds and are unable to diffuse or self-assemble into ordered
structures at room temperature [161, 181], while on the H-passivated surface
they diffuse freely and can form islands [181, 192]. The effect of the Ag(111)
surface on the NiDPP self-assembly is expected to be minor, similar to that of
H-passivated Si(111), however the Ag/Si(111)-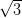 ×
×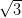 R30° surface’s electron
states have been shown to exhibit a resonance close to the energy of the dz2
molecular orbital [193], leading to a stronger interaction with the surface, and the
difference between the interactions with these two surfaces will be explored in this
chapter.
R30° surface’s electron
states have been shown to exhibit a resonance close to the energy of the dz2
molecular orbital [193], leading to a stronger interaction with the surface, and the
difference between the interactions with these two surfaces will be explored in this
chapter.