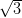 ×
×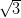 R30°.
R30°.
The interplay between the role of intermolecular forces and molecule–surface interactions is a very fine balance. The relative strengths of these two factors determines how molecules self-assemble when placed on the surface.
The effect of the molecular/inorganic interface on possible future device performance cannot be overstated, as this will determine the charge injection and charge flow in such molecular devices [76–78].
In this chapter, using STM and LEED, the effect of the substrate on the
self-assembly of a particular porphyrin molecule, (5,15-diphenylporphyrinato)Ni(II)
(NiDPP) is examined by depositing it onto two related – but different – surfaces,
Ag(111) and Ag/Si(111)-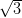 ×
×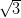 R30°.
R30°.
When deposited onto the less-reactive noble Ag(111) surface, NiDPP is able to diffuse across the surface to the step edges and grow into close-packed monolayers. These monolayers are composed of a single domain which covers the entire sample surface.
In contrast, when deposited on the more reactive Ag/Si(111)-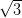 ×
×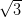 R30°
surface, the molecules adopt one of three preferential orientations, and bind to the
middle of terraces. These initially-bound molecules act as nucleation sites for
domains which grow across the terraces. Many domain boundaries are
evident on the surface, with all three orientations present on a single
terrace.
R30°
surface, the molecules adopt one of three preferential orientations, and bind to the
middle of terraces. These initially-bound molecules act as nucleation sites for
domains which grow across the terraces. Many domain boundaries are
evident on the surface, with all three orientations present on a single
terrace.
The results contained herein shed light on the complex interactions between adsorbates and surfaces, and show that the reactivity of the substrate plays a major role in molecular self-assembly.