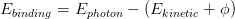
Similar to XAS, X-ray photoemission spectroscopy (XPS) involves a collimated, monochromatic beam of X-rays incident on a sample, however in this case, the kinetic energy and number of photoelectrons which escape the surface are measured. Due to the small mean free path of electrons in a material, XPS is a surface-sensitive technique, as only electrons ejected from the top 1–10 nm of a sample can be detected.
The photoelectric effect has a rich history, beginning with its discovery in 1887 by Heinrich Hertz, and its Nobel prize-winning explanation by Albert Einstein in 1905. Several attempts were made to make use of photoelectrons produced by X-rays, however it was only after World War II that Kai Siegbahn developed the equipment necessary for high energy resolution experiments, and, together with engineers from Hewlett Packard, produced the first commercial XPS instrument in 1969. His efforts to develop XPS into a useful tool for science were recognised in 1981 when he shared the Nobel prize in Physics.
An XPS spectrum is generally plotted as the number of electrons detected vs. their binding energy (Ebinding):
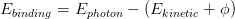
The relative binding energies of the electrons captured depend on their atomic orbitals, with core electrons having a higher Ebinding than weakly-bound outer-shell electrons, however the particular spectrum of energies observed is characteristic of the element being analysed. The number of electrons counted at a particular element’s characteristic energy is directly proportional to the amount of that element within the area irradiated (when a relative scaling factor is taken into account), and so XPS can give a quantitative measure of a sample’s make-up.