As has been touched upon in previous sections, some molecules appear to switch between “dim” and “bright” states [25, 35]. This phenomenon has previously been attributed to C60-induced substrate reconstructions [25, 30–36]. Such surface reconstructions lead to two topographically-different C60 adsorption sites, where dim C60 molecules are sunk into nanopits on the reconstructed substrate, and are lower in height than the bright ones [25, 30–36, 46]. The switching between dim and bright C60 states was attributed to changes in the substrate underneath the molecular layer due to the diffusion of substrate atoms [25, 35]. Other explanations suggest that this apparent height difference is due to electronic and molecular orientation effects [106, 107, 118]. Better understanding of the switching of C60 between different charge states is central to further progress in electronic devices utilizing these molecules.
This section describes the charge-transfer induced switching of some isolated C60 molecules within the monolayer grown on the WO2/W(110) surface. Such switching of individual C60 between different charge states represents a phenomenon that is different from the “bright” and “dim” contrast shown in Figure 3.10a, which was due to the “dim” molecules lying in the grooves between the nanorows. Furthermore, these two phenomena coexist on the WO2/W(110) surface. Our results indicate that C60 switching between charge states correlates with the molecule’s rotation, i.e. some orientations of the molecule favour its charge neutral state and others favour the negatively charged state.
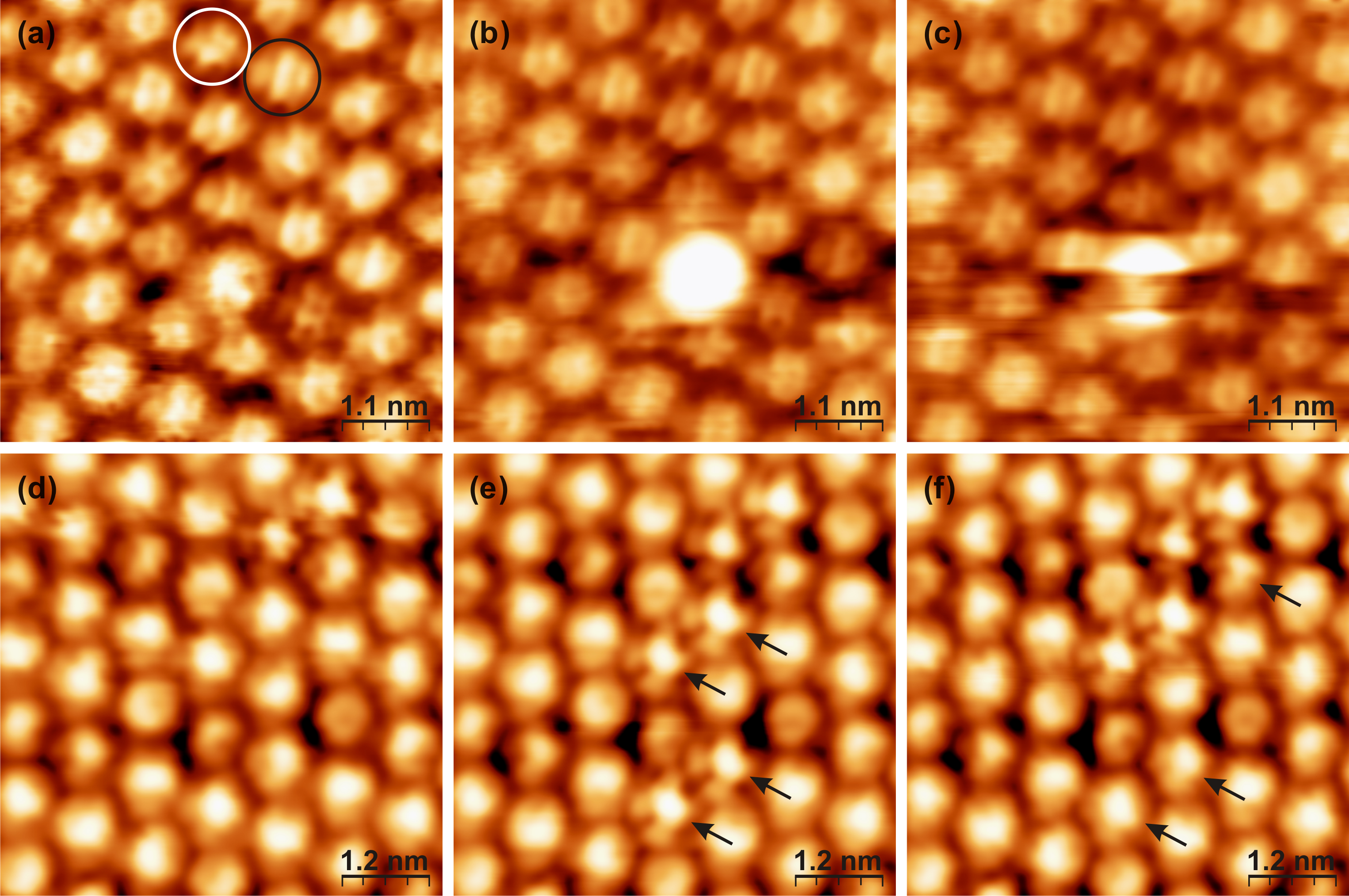
Figures 3.19a–c show a sequence of constant-current STM images of the same area of the C60 monolayer self-assembled on the WO2/W(110) surface, acquired at a sample bias of 1.0 V. These images, taken at 255 K, exhibit an internal structure corresponding to molecular orbitals for most of the C60 molecules. Comparing their appearance with the partial charge density calculated by DFT [46, 47] (Section 3.5.3), it is noted that the majority of the molecules face the substrate with an h–h bond (indicated by a black circle in Figure 3.19b), exhibiting three distinct “stripes” [25, 31, 32, 46, 47, 161]. Most of the other molecules face the substrate with an h–p bond (white circle). This is in agreement with DFT calculations described in Section 3.5.3 which show that the h–h orientation has the lowest total energy, while the h–p orientation differs from the lowest energy state by 17 meV [47], which is comparable to kBT in our STM experiments.

Although individual C60 molecules do not appear identical to one another due to their different orientations on the surface [25, 30–36], the orbital structure of each molecule remains unchanged from one image to the next, indicating that these molecules are not rotating. However, some individual molecules, such as the one at the centre of the image, switch between different states. In the temperature range between the phase transitions (220–260 K) such molecules change their appearance continuously in a random fashion for the entire duration of the experiment. Figure 3.19c demonstrates the case when switching occurs fast enough to be seen on a single image. This phenomenon is observed at both positive and negative biases, with the best apparent contrast achieved at a positive sample bias of 0.8 V.
Figures 3.19d–f show a sequence of constant-current STM images of another area of the monolayer, acquired at a sample bias of -1.9 V. Several molecules are observed to switch between scan frames (indicated by arrows). The apparent height difference between the molecules in the two different states is much smaller at this bias. The orientation of most of the molecules cannot be resolved at a negative bias. However, the orbital structure of the molecules in the state which has a lower apparent height is more pronounced and they are observed to be in the h–p orientation on the surface. It is noted that imaging the surface with a sample bias in the range of -0.5 V to 0.6 V, was unsuccessful, resulting in noisy images. This was most likely due to the band gap of C60. At lower temperatures the molecular switching between the different states ceases and at high temperatures the molecules rotate continuously so fast that no switching is observed and all molecules appear as perfect spheres [47].
Figure 3.20a shows a constant-current STM image of the C60 monolayer, acquired at 257 K. The image emphasizes the difference between the previously described phenomenon of the “bright” and “dim” C60 contrast [25, 30–36] and the C60 switching between charge states. Three types of molecules are clearly visible in Figure 3.20a: “dim”, “normal” and “very bright”. They are different in apparent height, which can be seen from the line profile taken along the dashed line in Figure 3.20a and shown in Figure 3.20b. The first two types represent “dim” and “bright” contrast due to the substrate topography, with “dim” C60 molecules arranged in dark chain-like structures on the surface (marked by the arrows in Figure 3.20). Such a contrast is also observed at temperatures below 220 K as well as at both bias polarities [46].
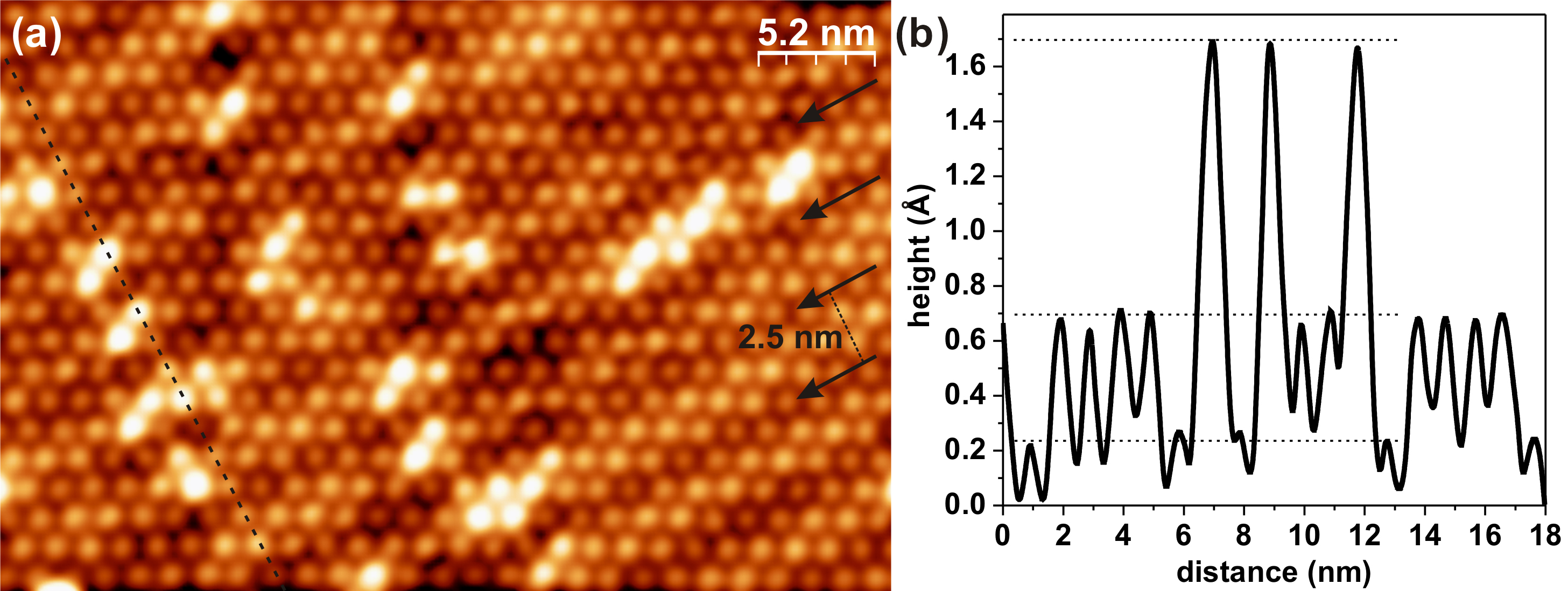
The distance between these chains is 2.5 nm, which is equal to the width of oxide nanorows forming the substrate [46]. These “dim” C60 molecules follow the oxide nanorows along the [37] direction of the substrate, occupy the grooves between them, and, as a result, are situated slightly lower (by approximately 0.5 Å, see Figure 3.20b) than the “normal” C60. There are, however, very bright molecules in Figures 3.19 and 3.20, with a much greater height difference of about 1 Å (Figure 3.20b) between them and the “normal” C60. These very bright molecules are predominantly located along the top of the oxide nanorows, with very few of them found lying in the grooves between the rows, as can be seen in Figure 3.20a.
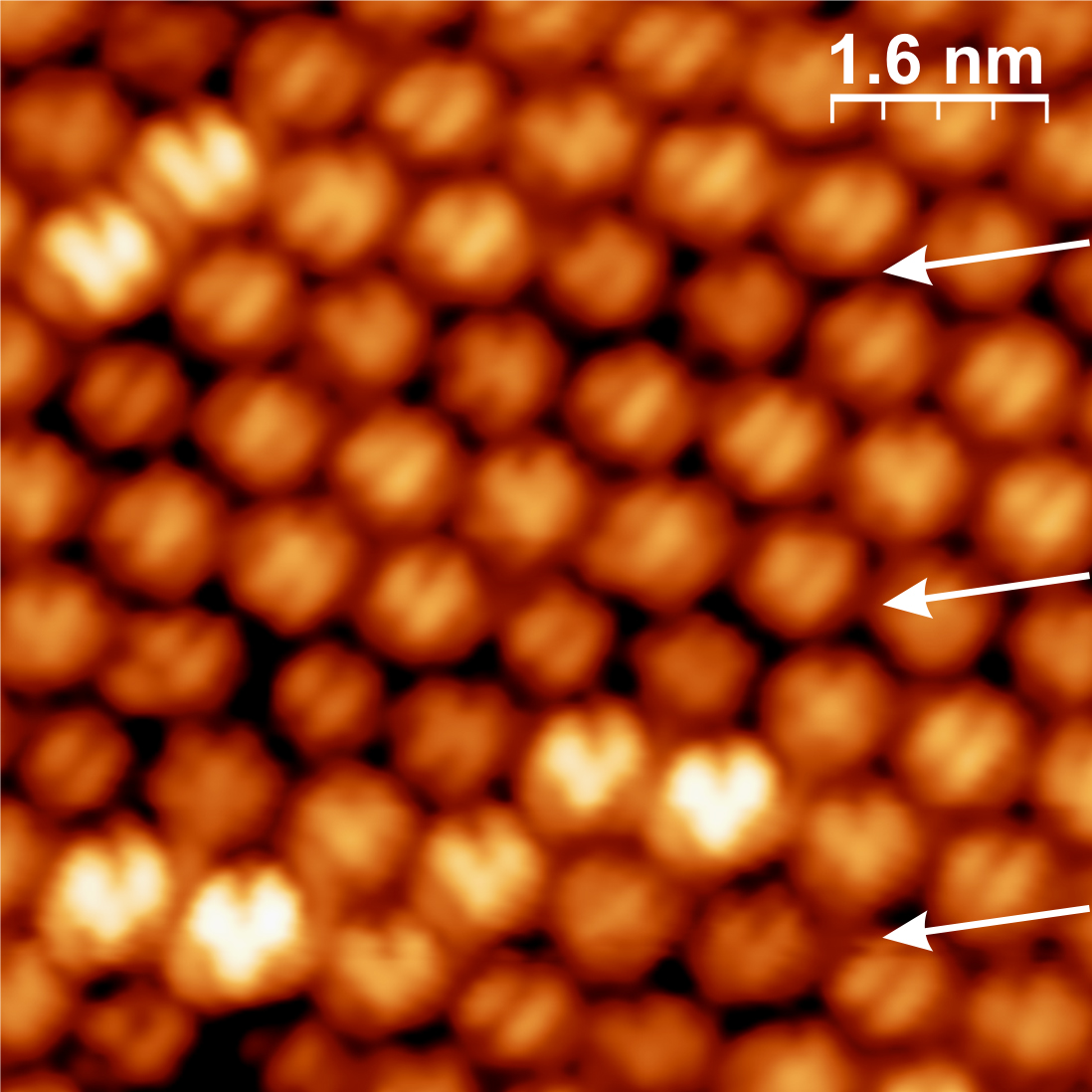
A majority of very bright molecules face the substrate surface with an h–p bond, as can be seen in Figure 3.21. Furthermore, such C60 molecules change their appearance in time, switching randomly between two different states. The rate of switching between the states depends on temperature. In the temperature range of 220–260 K, where switching is observed, the total number of switching molecules rapidly decreases with decreasing temperature.
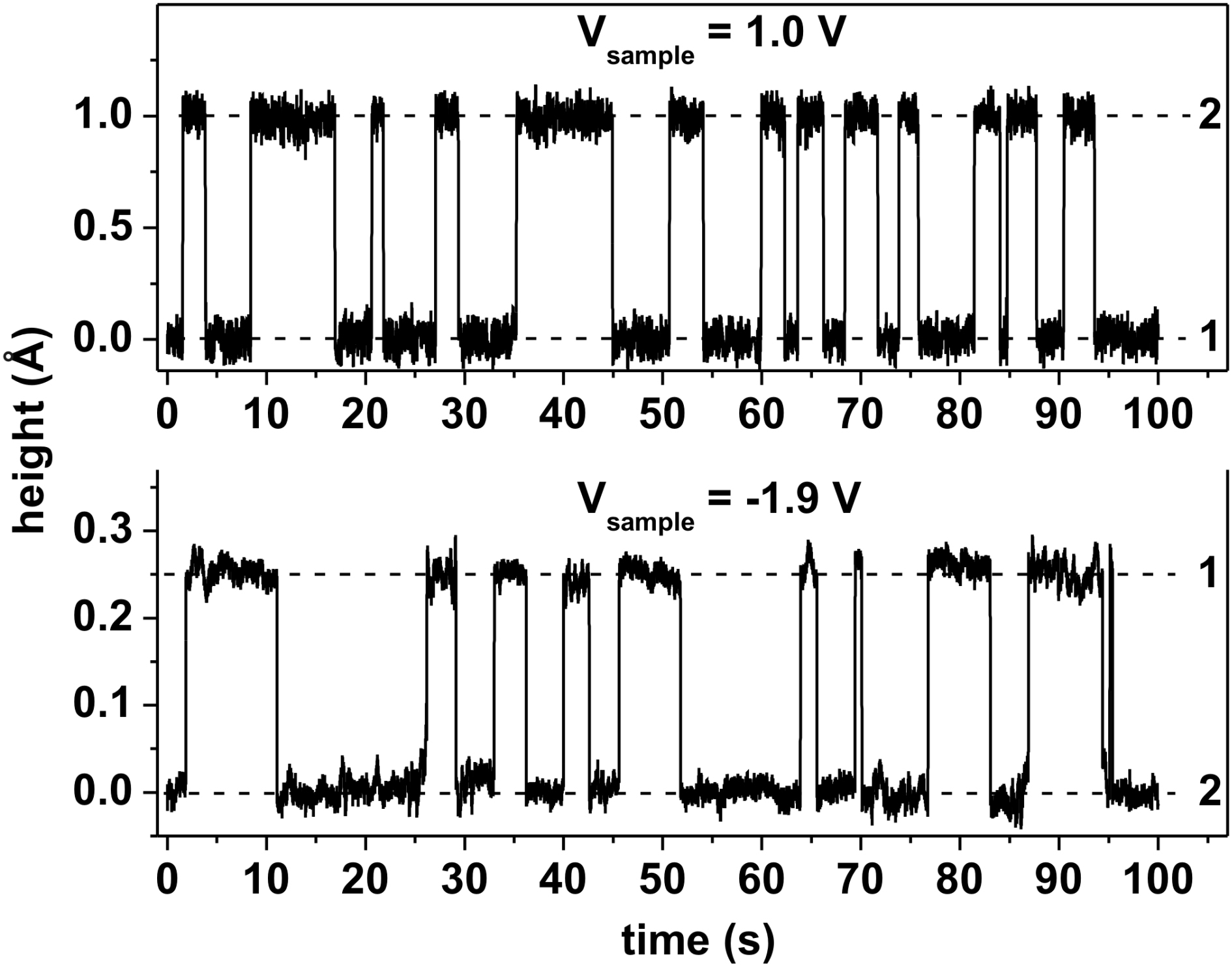
The time-evolution of the tip-surface distance measured at two different sample biases (1.0 V and -1.9 V) is presented in Figure 3.22, when the STM tip is placed above an individual switching C60. In both cases the STM tip shifts between two different height levels (indicated by dashed lines), corresponding to two different states of the molecule. The tip-surface distance changes by approximately 1 Å during the switching of the molecule when a bias of 1.0 V is applied (Figure 3.22, top spectrum). This cannot be explained in terms of different geometrical orientations of the C60 on the surface or by C60-induced substrate reconstructions [25, 30–36].
If, for example the C60 molecules were inducing the substrate to reconstruct, the number of bright molecules would be conserved over time, as described by Gardener and co-workers [25]. However in the present case, molecules are observed to switch at random. The quality of the oxide substrate was confirmed by STM prior to C60 deposition, and the film was found to have a very low concentration of defects. Since C60 switching was observed over the entire sample and was not limited to one particular area, it can be concluded that the effect is not caused by the presence of defects.
It is suggested that this C60 state arises due to charging of the molecule, which causes changes in the local density of electron states and consequently a variation in tunneling current. Using density functional theory calculations, it was found that the two different states of the C60 on the WO2/W(110) surface can be related to: (1) the charge-neutral C60 (normal appearance, Figure 3.19a) and (2) the negatively charged C60, which has accepted an electron (C− 60, very bright appearance, Figure 3.19b). It is noted that, in most cases, switching between the charge-neutral C60 in the h–h orientation and the C− 60 in the h–p orientation has been observed (see Figures 3.19d–f).
However, in rare events, more than two states were distinguished during the switching of some molecules, indicating that this phenomenon can be quite complex. It is proposed that the negatively charged C60 state results from the acceptance of a tunneling electron from the STM tip or the substrate depending on the bias applied. This is based on the fact that the switching of the molecule into the charged state is triggered continuously when the STM tip is static above an individual C60 with a bias applied (as in Figure 3.22). Molecular movement accompanies the molecule’s switching back to the charge neutral state, i.e., as the molecule rotates, it loses charge to the substrate or neighbouring molecules.
A similar charging process was observed by Repp et al., for the system of gold atoms adsorbed on bi- and trilayers of NaCl grown on the Cu(111) and Cu(100) surfaces [162]. By positioning the STM tip above an Au adatom and applying a voltage pulse, the adatom can be reversibly switched between its neutral and negatively-charged state. Both states have been found to be stable due to the greatly suppressed mobility of Au adatoms at the temperature of the experiment (5–60 K) as well as the reduced coupling of the electronic states of the adatom with the metal substrate due to the insulating NaCl film [162]. Recently, it has been shown that the charge state of molecules can be manipulated in a similar manner [163].
STM experiments performed at temperatures below 10 K have revealed that individual Cu phthalocyanine molecules deposited on the NaCl/Cu(111) surface can be negatively charged by applying low voltage pulses [163]. In our experiment, the ultrathin WO2 layer reduces the coupling of the electronic states of the C60 with the W(110) substrate, which allows the molecule to hold charge for some time. However, each C60 molecule that undergoes switching is surrounded by six neighbours in the monolayer, resulting in van der Waals interactions between the molecules. This interaction may influence molecular movement and stability on the surface and hence affect the switching.
Figure 3.23 shows the density of states (DOS) calculated for two different molecules, the charge-neutral C60 and the C− 60 with the h–h and h–p orientations, respectively. The C60 orientation on the surface in each case has been chosen on the basis of experimental observation that most of the “normal” molecules face the substrate with an h–h bond, while the majority of very bright C60 face the substrate with an h–p bond. The addition of an electron to the C60 molecule changes its electron density of states. This results in the old lowest unoccupied molecular orbital (MO) being half-occupied and becoming the new highest occupied MO, which in turn causes the valence band structure to shift relative to the Fermi energy.
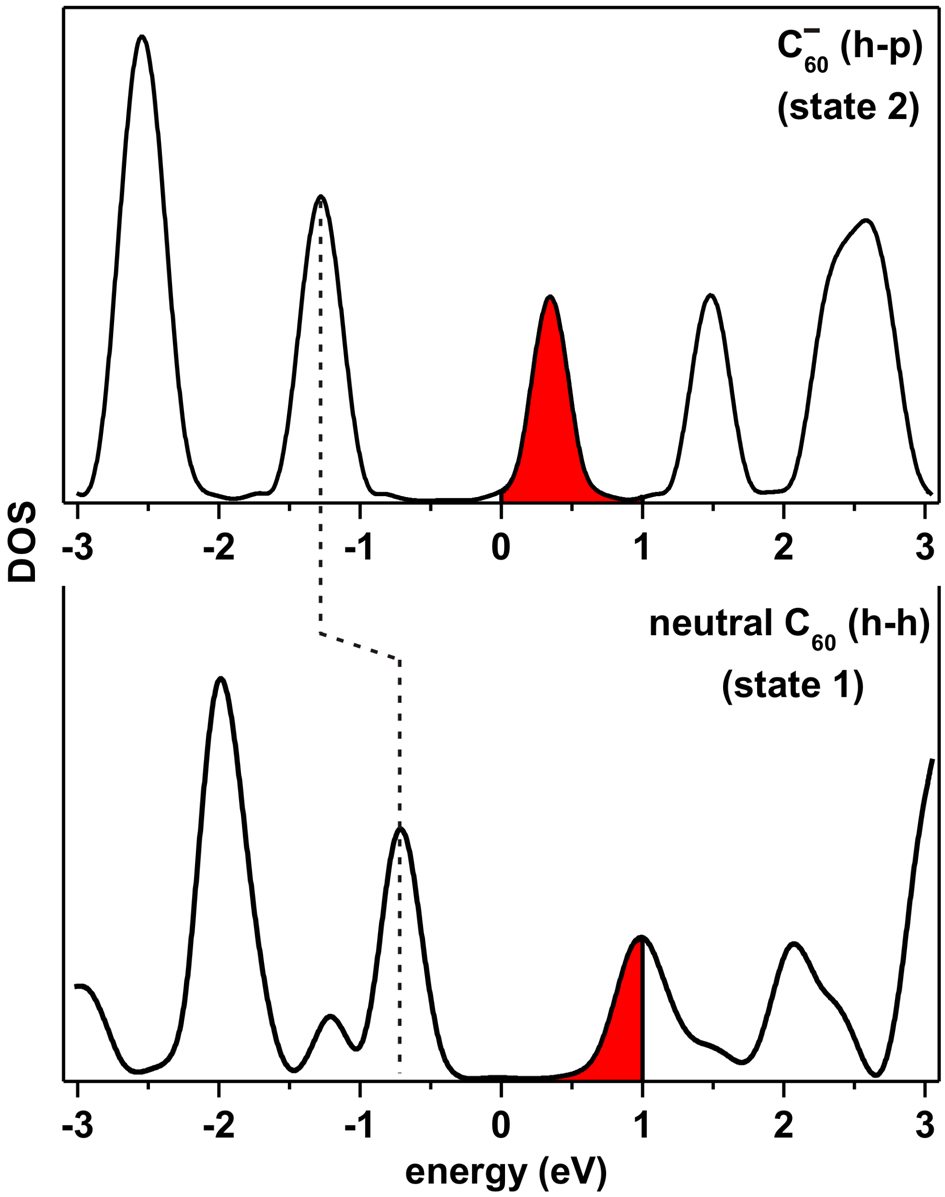
The highlighted area in Figure 3.23 shows the molecular states involved in tunneling, when a 1.0 V sample bias is applied. This bias leads to the apparent height contrast of 1.0 Å between different molecular states in STM images (see Figures 3.19 and 3.20) and corresponding changes in the STM tip-surface distance (Figure 3.22, top curve). Note that there is a significant increase in the molecular states available for tunneling (in the energy range from 0–1.0 eV) in going from the charge-neutral C60 molecule (state 1) to the C− 60 (state 2), which is reflected in the very bright appearance of the latter at the 1.0 V sample bias.
A difference in DOS for the C− 60 and C60 molecules (Figure 3.23) suggests that the apparent height difference ΔZ should depend on the applied bias voltage. Indeed, at sample biases higher than 1.5 V (or lower than -1.5 V), the observed apparent contrast between different C60 states was significantly smaller because multiple molecular orbitals were involved in tunneling. This minimizes the difference between the DOS for each state of the C60 characterised by the molecule’s charge and orientation on the surface.
From Heisenberg’s uncertainty principle, ΔEΔt > ℏ∕2, the energy resolution of a state should be inversely proportional to its lifetime [164], i.e. an electron should only exist for a short time in a state whose energy range is broad. This would imply that the broad states calculated in Figure 3.23 should only lead to an observed lifetime on the order of femtoseconds, however one must remember that for the system in question, charge transfer is not the only phenomenon that must be accounted for. In going from the neutral C60 to charged C− 60, the molecule must rotate from h–h to h–p, which will have its own associated characteristic timescale, and the presence of the STM tip causes charging to be constantly triggered by tunneling electrons. The tip itself may also affect the rotation of the molecule, thus the full complexities of the switching mechanism are not encompassed by simply calculating the DOS for the two “final” states, however it is illustrative in explaining the apparent contrast between the two observed states. It is unfortunately beyond the scope of this thesis to develop a full theoretical basis for the switching mechanism, however it is an interesting avenue for future work.
The STM tip-surface distance changes by only 0.25 Å during the switching of the molecule with -1.9 V bias applied (Figure 3.22, bottom curve). This is due to the relatively small difference between the molecular states of C60 and C− 60 available for tunneling between -1.9 eV to 0 eV. At this bias the C− 60 molecule (state 2) has a slightly darker appearance in STM images than the charge neutral C60 (Figures 3.19d-f), which is in good agreement with the STM tip-surface distance obtained at -1.9 V bias (Figure 3.22, bottom curve).
In constant current mode, the apparent height difference ΔZ between the C− 60 and the C60 molecules can be determined as:
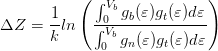 | (3.1) |
where gb(ε) and gn(ε) are the DOS of the C− 60 and C60, respectively, gt(ε) is the DOS of the STM tip and k = 2Å -1 [165]. The DOS of the monocrystalline W(100) tip, previously calculated in this group [74, 166], exhibits no major features close to the Fermi energy and can be treated as nearly constant in the range considered here. Although it cannot be guaranteed that the tip does not have an attached molecule during the experiments, STM performed before and after z(t) measurements were consistent with the most stably-observed appearance of the film, and so it is assumed for simplicity that the tip apex consists of only clean tungsten.
Using this formula, the ΔZ between the C− 60 (h–p orientation) and the C60 (h–h orientation) molecules on the WO2/W(110) surface has been calculated from the density of states presented in Figure 3.23 under the assumption that gt(ε) is nearly constant and is shown in Figure 3.24 as a solid line. This curve is in good agreement with the experimental data for ΔZ obtained from STM images and the time-evolution of the tip-surface distance measured at different sample biases. The data presented in Figure 3.24 indicate that both a large value of ΔZ at certain biases and its overall dependence on bias voltage can only be explained in terms of different charge states of the C60 molecules on the surface.
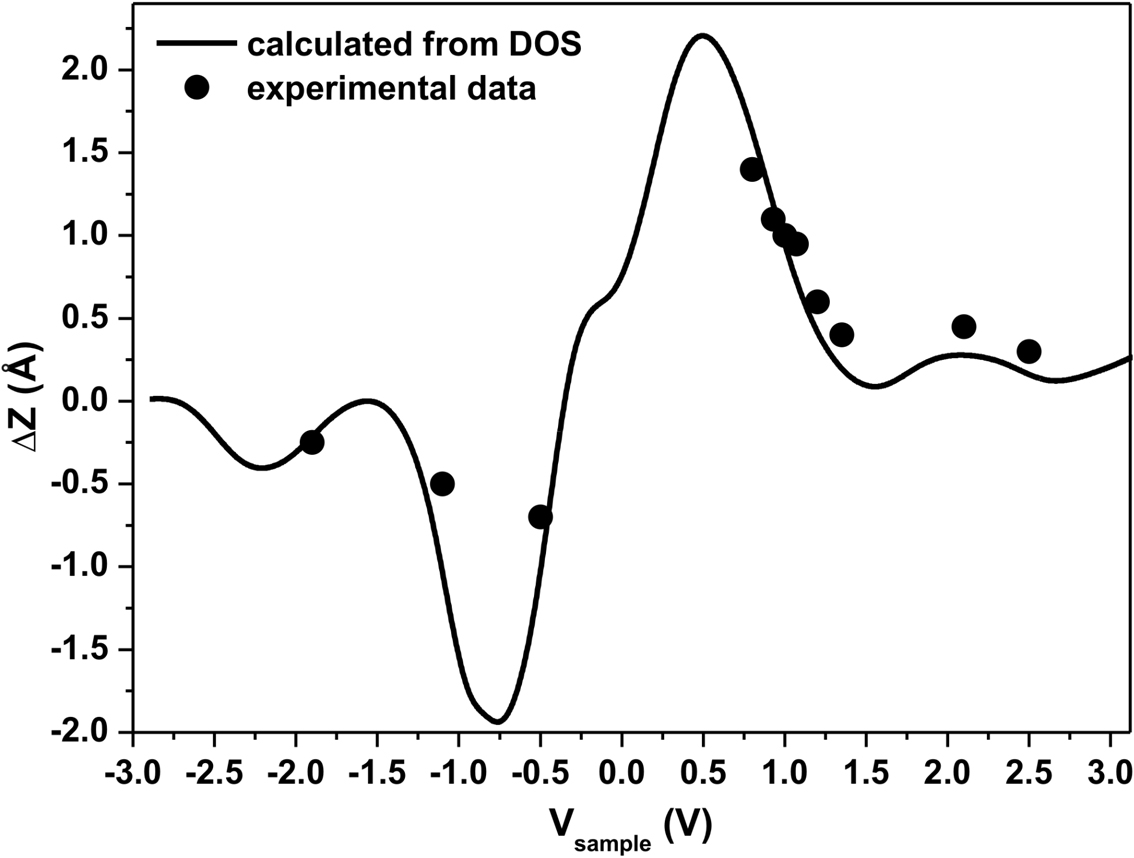
These observations are in good agreement with the results of Yamachika et al.,
obtained during their low-temperature study of controllable C60 doping with K
atoms on the Ag(001) surface [167]. They have found that approximately 0.6 of
an electron is transferred to C60 from each K. They have observed only small
changes in apparent size between the C60 and K4C60 molecules when scanned at
2.0 V sample bias. At this bias the number of orbitals involved in tunneling does
not change dramatically when going from C60 to K4C60, as observed by scanning
tunneling spectroscopy [167]. However, the 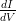 map measured at 1.25 V and 50 pA
was found to be much brighter in the case of the K4C60 molecule, where
approximately 2.4 electrons are transferred to the C60, than in the case of
undoped C60 [167].
map measured at 1.25 V and 50 pA
was found to be much brighter in the case of the K4C60 molecule, where
approximately 2.4 electrons are transferred to the C60, than in the case of
undoped C60 [167].
It has been observed that switching C60 molecules change their orientation on the surface. Furthermore, most of the “normal” molecules face the substrate with an h–h bond, while the majority of negatively charged C60 face the substrate with an h–p bond. It is reasonable to propose that the process of gaining or losing an electron depends on the C60 orientation. Charge transfer efficiency to or from the molecule depends strongly on which part of the molecule is most reactive. The spatially-resolved reactivity of the molecule can be visualized by the Fukui function, f(r), which is considered as a convoluted reactivity indicator [168].
The Fukui function (f+(r) or f−(r)) is defined as the differential change in the electron density due to an infinitesimal change (increase or decrease) in the number of electrons. When the molecule accepts electrons, the electrons tend to go to the part of the molecule where the Fukui function f+(r) is large, because at these locations the molecule is most capable of stabilizing additional electrons [168].
Figure 3.25 shows cross sections of f+(r) and f−(r) taken along the h–h and h–p bonds through the centre of the C60, calculated using Dmol3 (Accelrys Materials Studio 4.3). The value of f+(r) (centre panel) is larger for the h–p bond with respect to the h–h bond, in agreement with previous calculations [168]. This indicates that the addition of electrons to C60 is more effective when an h–p bond of the molecule faces the WO2/W(110) surface and, due to the molecule’s symmetry, the STM tip.
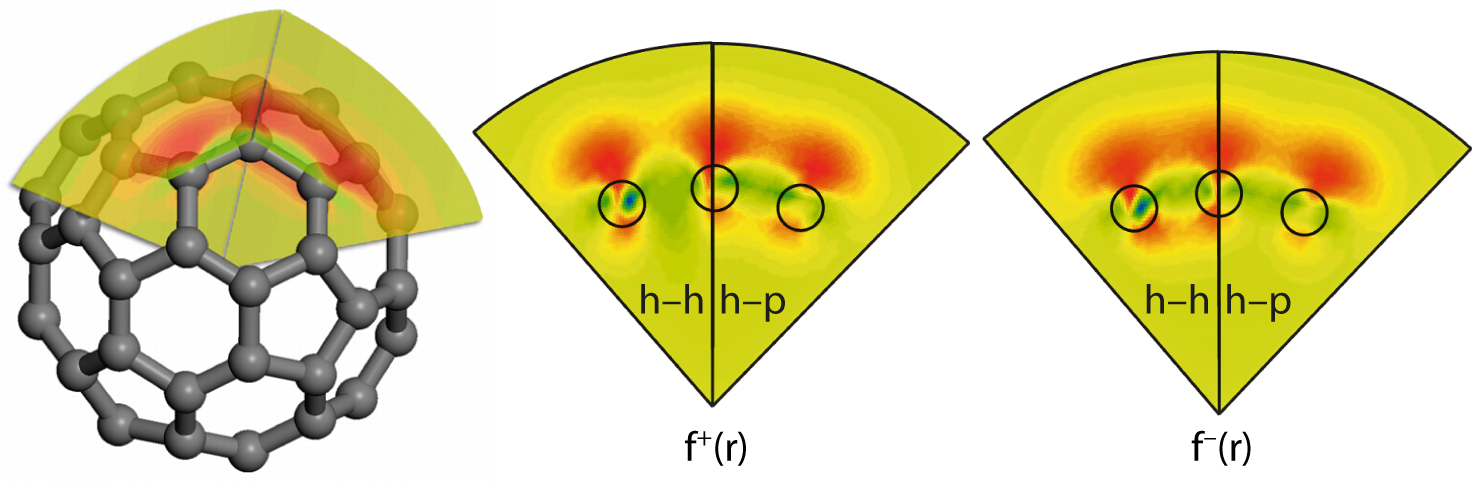
In turn, the analysis of f−(r) (Figure 3.25) allows one to conclude that the removal of an electron occurs more effectively via h–h bonds. Thus a strong correlation between the charge state of the C60 molecule and its orientation on the surface observed in experiments can be explained in terms of charge transfer efficiency. Electron acceptance by the molecule is facilitated through its rotation to achieve an h–p orientation, which is the most suitable for such charge transfer. In turn, electron loss is accompanied by the molecule’s rotation back to an h–h orientation.
Combining all the findings, the following mechanism for the C60 switching on the WO2/W(110) surface can be proposed. When the molecule in the neutral state is facing the surface by an h–h bond, there are four h–p bonds in the lower (or upper) part of C60 which are located in a symmetric way around this h–h bond. Due to thermal excitation of the molecule, one of these h–p bonds may come closer to the surface (or the STM tip) than the other three and accept an electron. This leaves the negatively charged molecule locked to the surface via this h–p bond. The ultrathin WO2 layer reduces the coupling of the electronic states of the C60 to the W(110) substrate, which allows the molecule to hold this charge for some time. However, each C60 molecule that undergoes switching is surrounded by six neighbours in the monolayer, resulting in van der Waals interactions between them. This interaction may influence molecular movement and trigger the loss of the electron. This view is simplified to highlight the correlation between the charging of C60 and its movement on the WO2/W(110) surface. There can be additional complexities one may need to take into account. For example, our calculation of the Fukui function was done for an isolated C60. The proximity of the molecule to the WO2/W(110) surface and to an STM tip will alter the Fukui function.