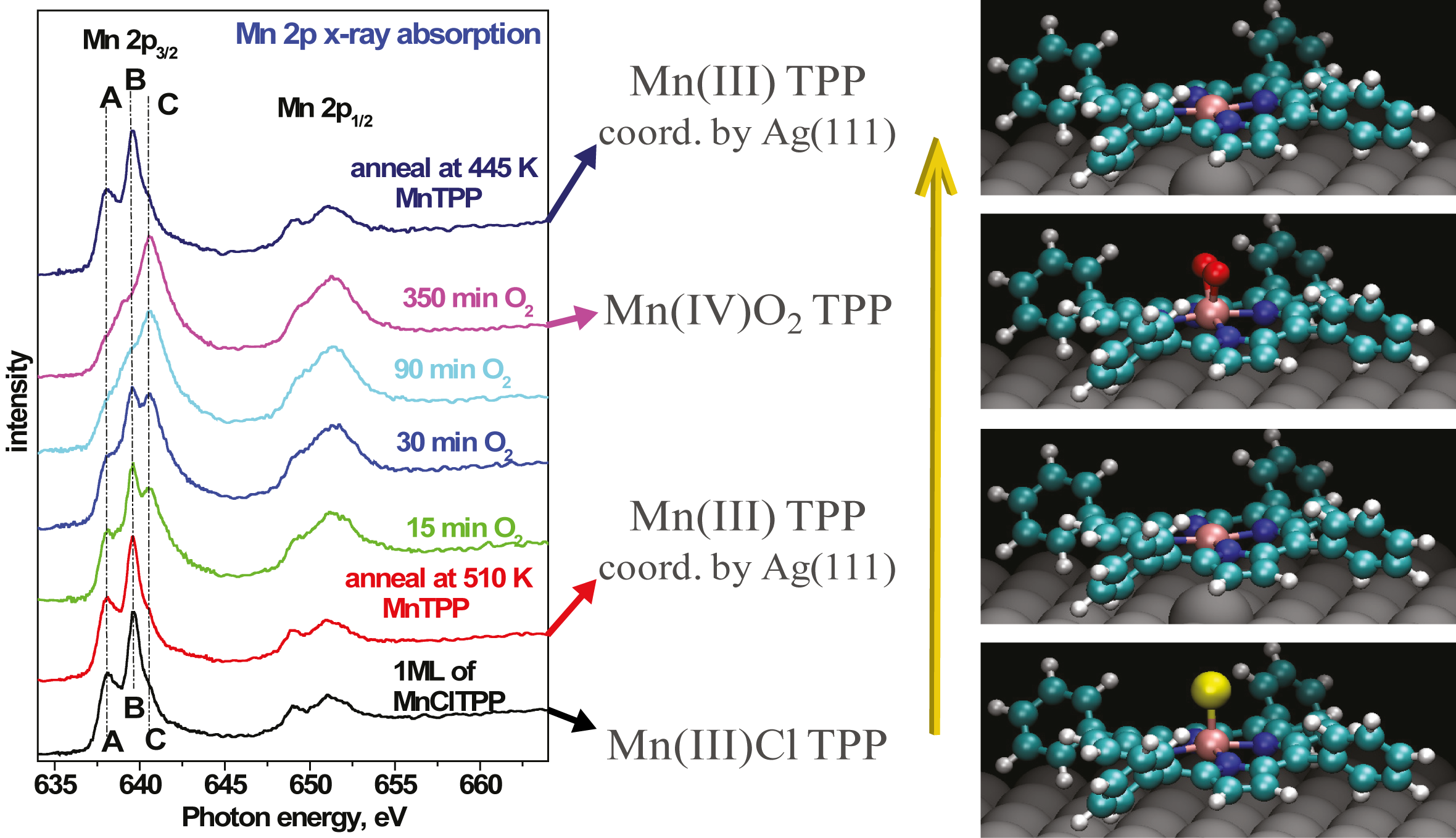
X-ray absorption spectroscopy (XAS) performed on the as-deposited MnClTPP exhibits the characteristic shape of the Mn(III) oxidation state, as shown in Figure 5.7 (bottom spectrum).

The Mn 2p3∕2 (L3) XAS edge consists of three features, labelled A, B and C in Figure 5.7. These correspond to a convolution of the interactions between the core 2p electrons with the outer valence electrons, and have been shown to reflect the oxidation state of the Mn ion [225, 226]. However, the decomposition of such spectra is complex, involving up to 7 individual peaks [227], and so the main features will be treated as a “fingerprint” of the oxidation state here. When the Mn ion is in the (III) oxidation state, the B feature dominates, with a significant contribution from A and a lower shoulder at C, as shown in the initial and final spectra in Figure 5.7. In the Mn(IV) state, the contribution of the C feature dominates.
When the Mn(III)ClTPP ML is annealed at 510 K, the Cl ligand is removed but from the shape of the XAS (red curve) it is clear the oxidation state of the Mn ion is unchanged. Without the Cl ligand, the Mn(III) ion would be undercoordinated by only the porphryin macrocycle alone. It is suggested that the Mn is coordinated by the Ag(111) surface, which acts as a fifth ligand for the Mn ion, stabilising the (III) oxidation state. A similar hypothesis has been put forward for previous studies of axial ligand transformation [195, 211, 228], with an atom of the noble metal surface ligating to the transition metal core.
Upon subsequent exposure of the Mn(III)TPP/Ag(111) complex to O2, the Mn 2p lineshape changes significantly. As oxidation time increases, the relative contributions of the A and B features diminish, and the C peak grows to be the principal feature. This implies that exposure to oxygen causes the Mn ion to change from the III oxidation state to the IV. Also noted are clear changes in the Mn 2p1∕2 peak, which are harder to quantify, but nevertheless support the oxidation of the Mn core.
It is postulated that the oxygen molecule binds to the Mn(IV) ion intact as the peroxide anion, O22−, with the two O atoms sharing a single bond and the anion bound to the Mn centre, and this will be shown in detail in the following sections. The Mn oxidation is reversible however, and further annealing at 445 K removes the bidentate peroxide ligand, restoring the Mn(III) lineshape.
STM performed after the initial anneal step to remove the Cl-ligand is presented in Figure 5.8. The occupied-state appearance of MnTPP (Figure 5.8a) appears almost identical to that of MnClTPP prior to annealing.
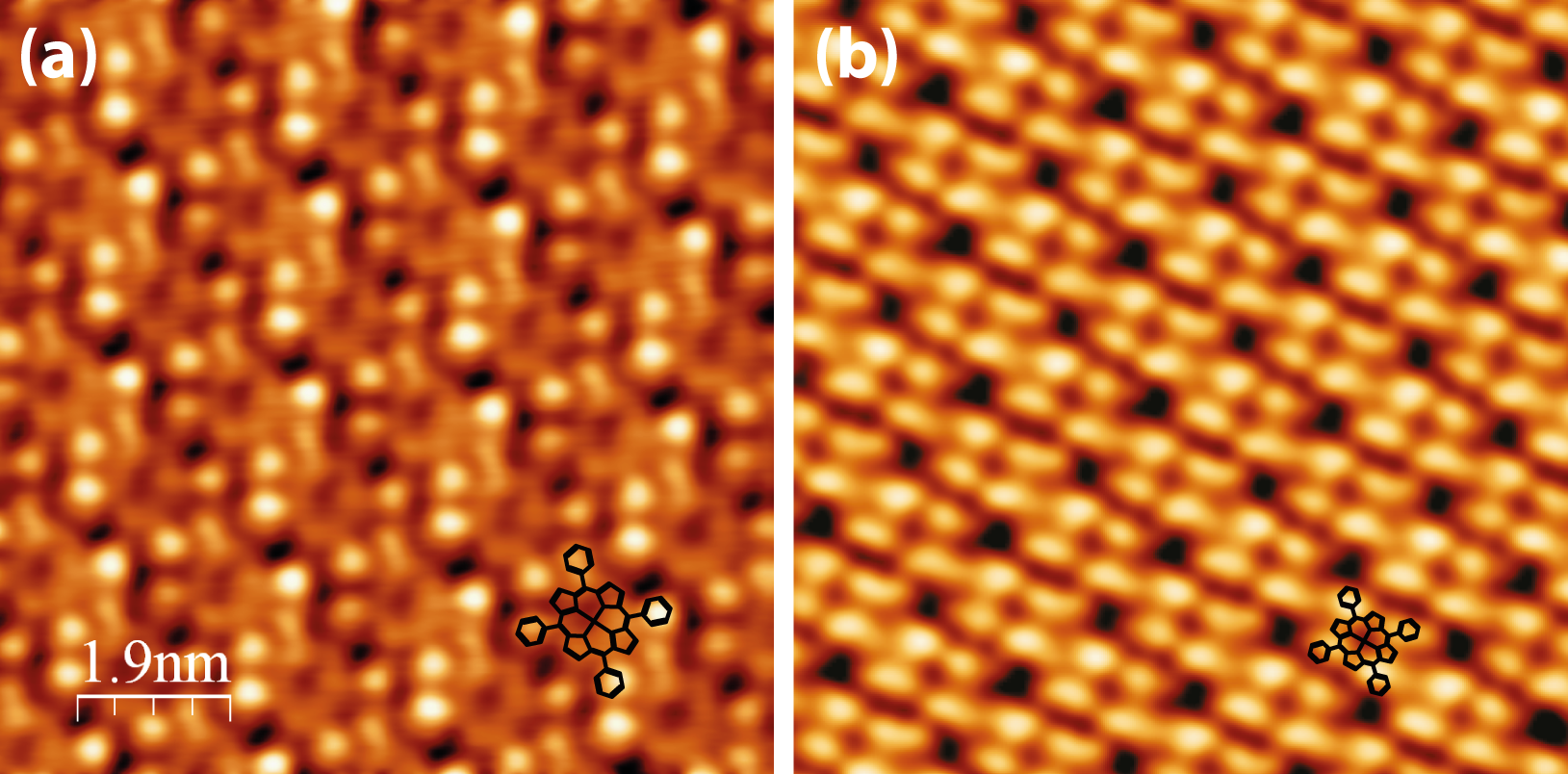
The four phenyl rings are still visible and the centre of the macrocycle appears dark, as in Figure 5.6a, and the monolayer exhibits unchanged square lattice parameters of 1.41 ± 0.05 nm. The rectangular arrangement of the phenyl rings is also unchanged, implying that the removal of the axial ligand does not greatly affect the aryl-group substituents. However, when the unoccupied states are probed at a positive sample bias (Figure 5.8b), the molecules’ appearance is strikingly different.
The central protrusion previously seen is gone, giving further evidence for the removal of the Cl-ligand. Instead, the molecules appear as four large protrusions, corresponding to the positions of the phenyl substituents. The “buckling” described in a previous section is still visible in both the occupied and unoccupied state images, suggesting that it is topological in nature (i.e. a rotation of one phenyl ring out of the surface), however in the latter case it is less pronounced.
In order to examine the behaviour of the MnTPP molecules after the Cl-ligand is removed, Mn(III)TPP on Ag(111) was simulated using DFT, and the relaxed model is shown in Figure 5.9, along with the relaxed model of Mn(III)ClTPP. In the simulation, four layers of Ag were used, with the lower two constrained to simulate the bulk. The molecules were placed on top of the Ag(111) surface and allowed to relax along with the top two layers of Ag. A vacuum slab of 10 Å was used to separate the system from its translational images.
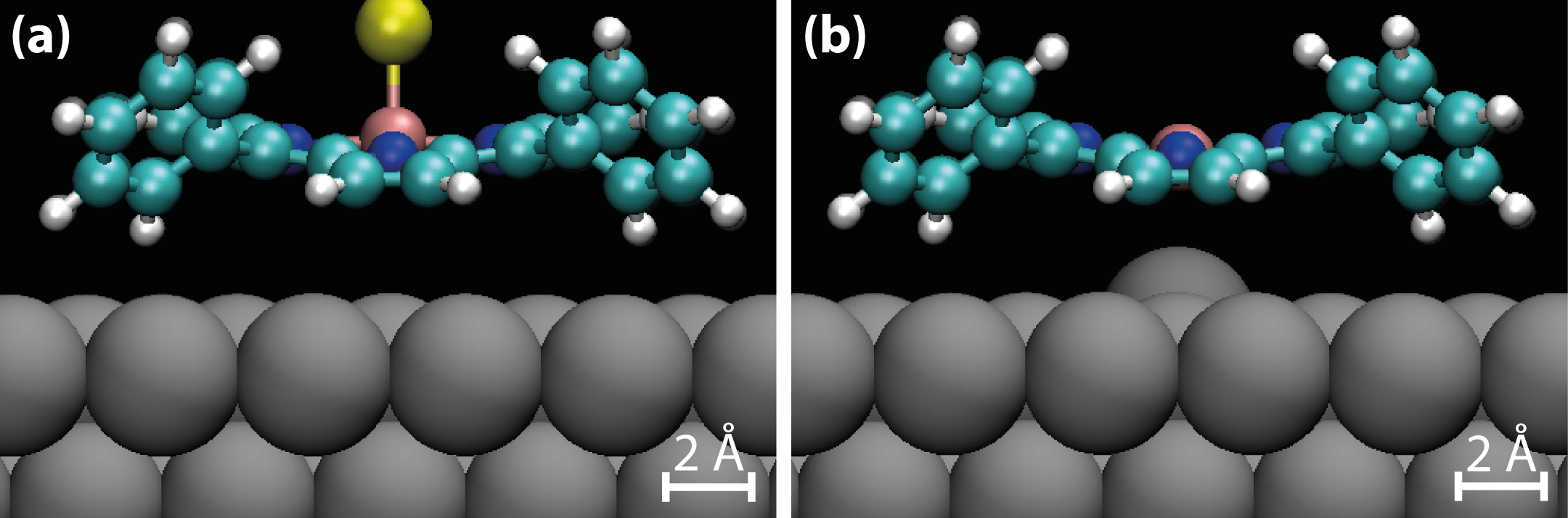
As can be seen from the side-views in Figure 5.9a and b, the removal of the Cl-ligand has little effect on the porphyrin macrocycle and its phenyl substituents, consistent with STM images.
The Mn centre in Mn(III)TPP, however, is situated 3.4 pm closer to the Ag(111) surface, and in turn the Ag atom directly below the Mn ion is pulled out of the surface towards the molecule by 8.7 pm. This supports the theory that the Ag(111) surface is indeed acting as a fifth ligand and stabilising the (III) oxidation state of the Mn ion centre.
In order to measure the activation energy required to trigger the desorption of the Cl-ligand, XPS was performed on the MnClTPP monolayer while it was annealed. The spectra obtained are presented in Figure 5.10a.
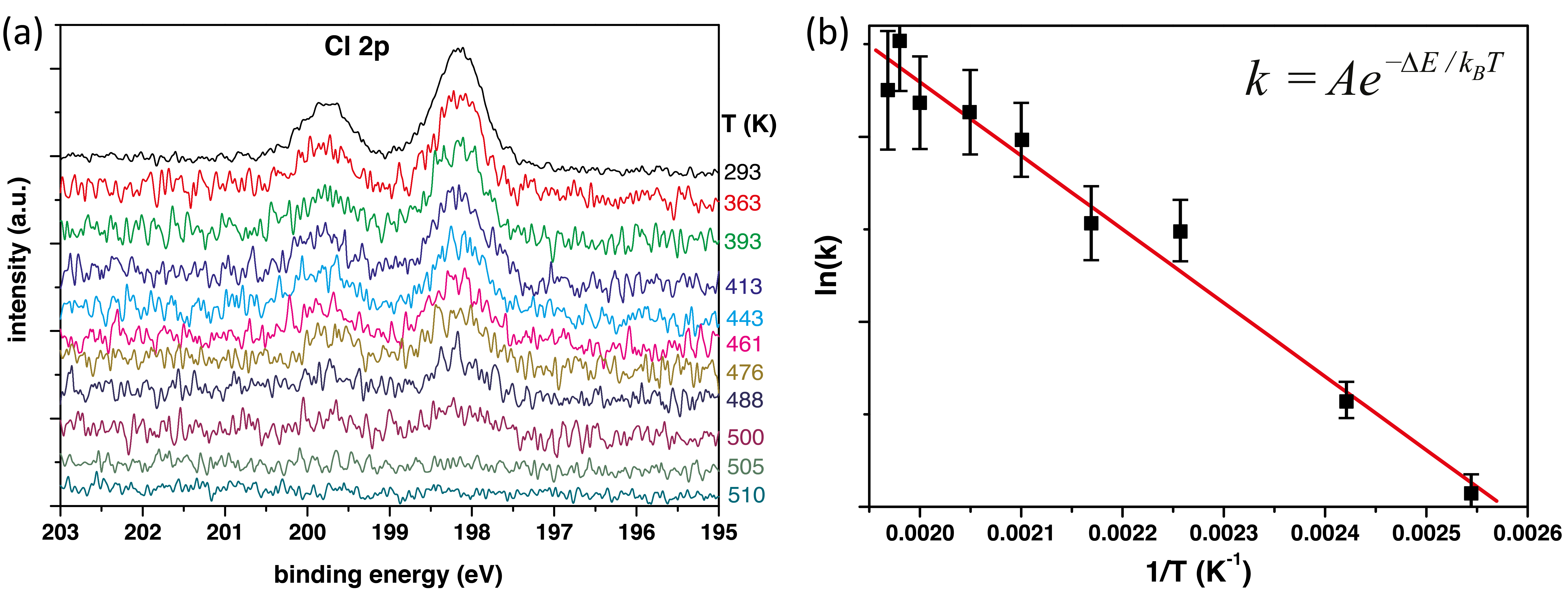
The spectra were measured while the temperature was increasing, with the temperature shown on the right of the plots. It is observed that at around 510 K, the Cl 2p peaks are reduced to the level of the background noise.
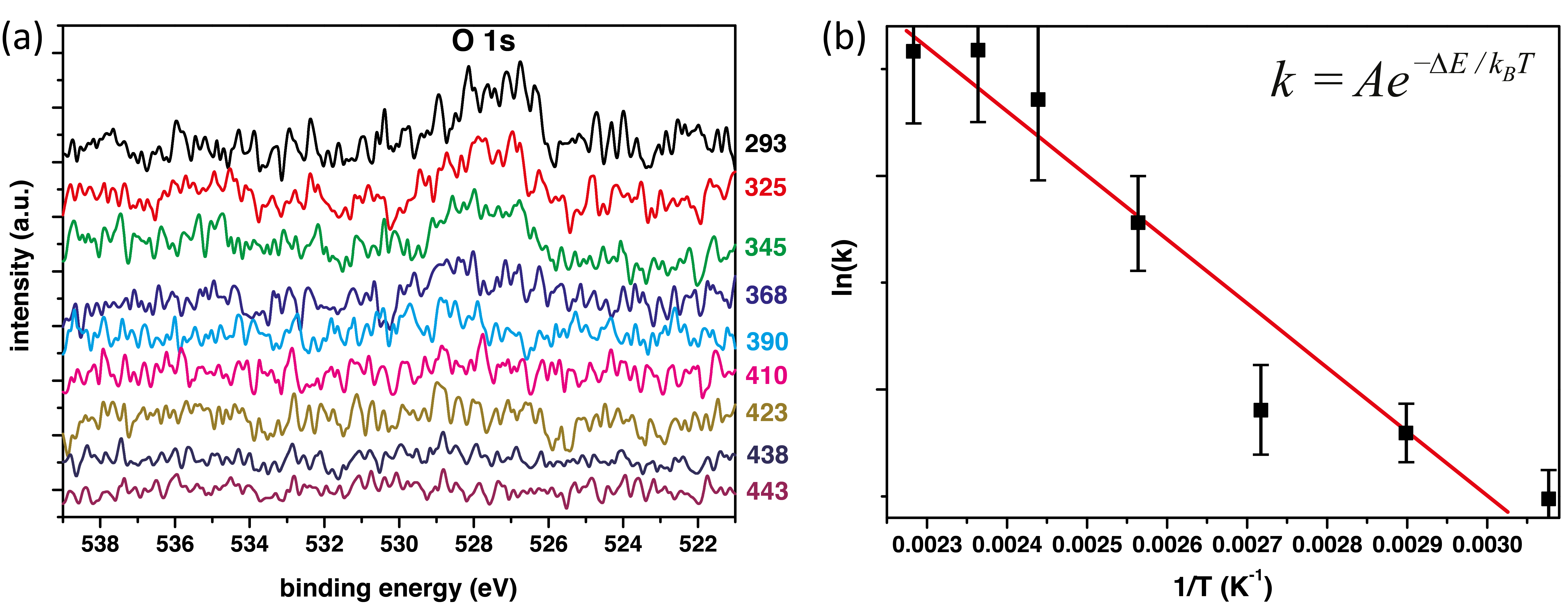
The Arrhenius rate equation (Equation 5.1) relates the rate of a reaction, k with its activation energy, ΔE and the temperature of the reaction, T.
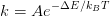 | (5.1) |
where kB is the Boltzmann constant and A is the prefactor.
The two Cl 2p peaks were each fitted by a Gaussian, and the area of that fit peak was was used to calculate the rate of Cl removal, taking into account the deviation of the spectrum from the fit to calculate the uncertainties. Since the experiment was performed with a constant heating rate of 0.15 K/sec, the difference in area between two spectra can be used to derive the rate of the desorption. ln(k) vs. 1∕T is plotted in Figure 5.10b, and the slope of the fit line gives a value for the activation energy of ΔECl = 0.35 ± 0.02eV.
After removing the Cl ligand from the MnClTPP molecules, they were exposed to oxygen gas for various lengths of time in order to study the effect of oxidation on the molecular layer.
STM images recorded after 15, 30 and 90 minutes’ oxygen exposure at a partial pressure of 2 × 10-6 mbar are shown in Figure 5.11. It is observed that some molecules exhibit a very bright centre, and the number of these molecules increases with oxygen exposure, so it is reasonable to assume that this appearance corresponds to the Mn(IV)O2TPP complex. The lattice parameters of the ML are unchanged from those of MnClTPP and MnTPP, consistent with observations that the meso-aryl substituents are the main factors in supramolecular ordering [194].
The percentages shown on each image indicate the amount of Mn(III)TPP molecules that have been converted to the Mn(IV)O2TPP state. The coverages correspond approximately to the XAS spectra shown in Figure 5.7, with the green spectrum showing ∼15% of the molecules oxidised, dark blue ∼40%, and light blue ∼80%.
After exposures of 90 minutes or more, approximately 80% of the molecules are converted to Mn(IV)O2TPP, however at this point the extent of oxidation appears to saturate. The ∼20% unoxidised molecules are attributed to those molecules that lie on top of defects on the surface, or the very few that still have the Cl-ligand attached.
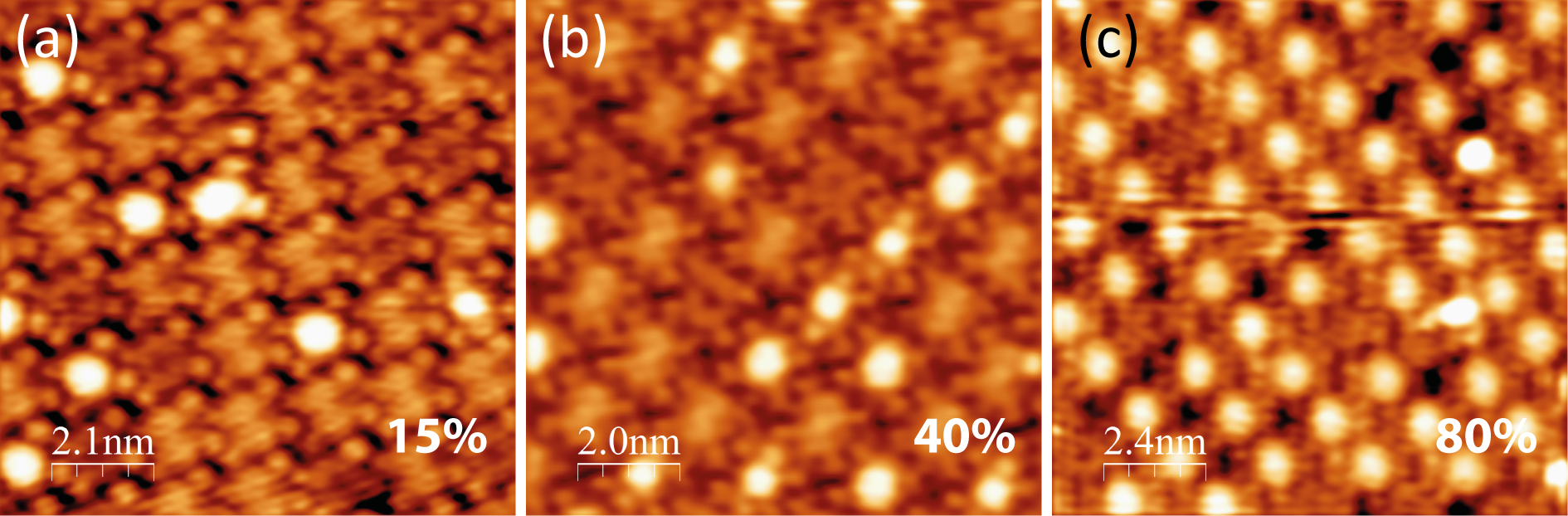
The MnO2TPP molecule was also simulated using DFT. Identical constraints were applied as in the previous section. The relaxed structure is shown in Figure 5.12.
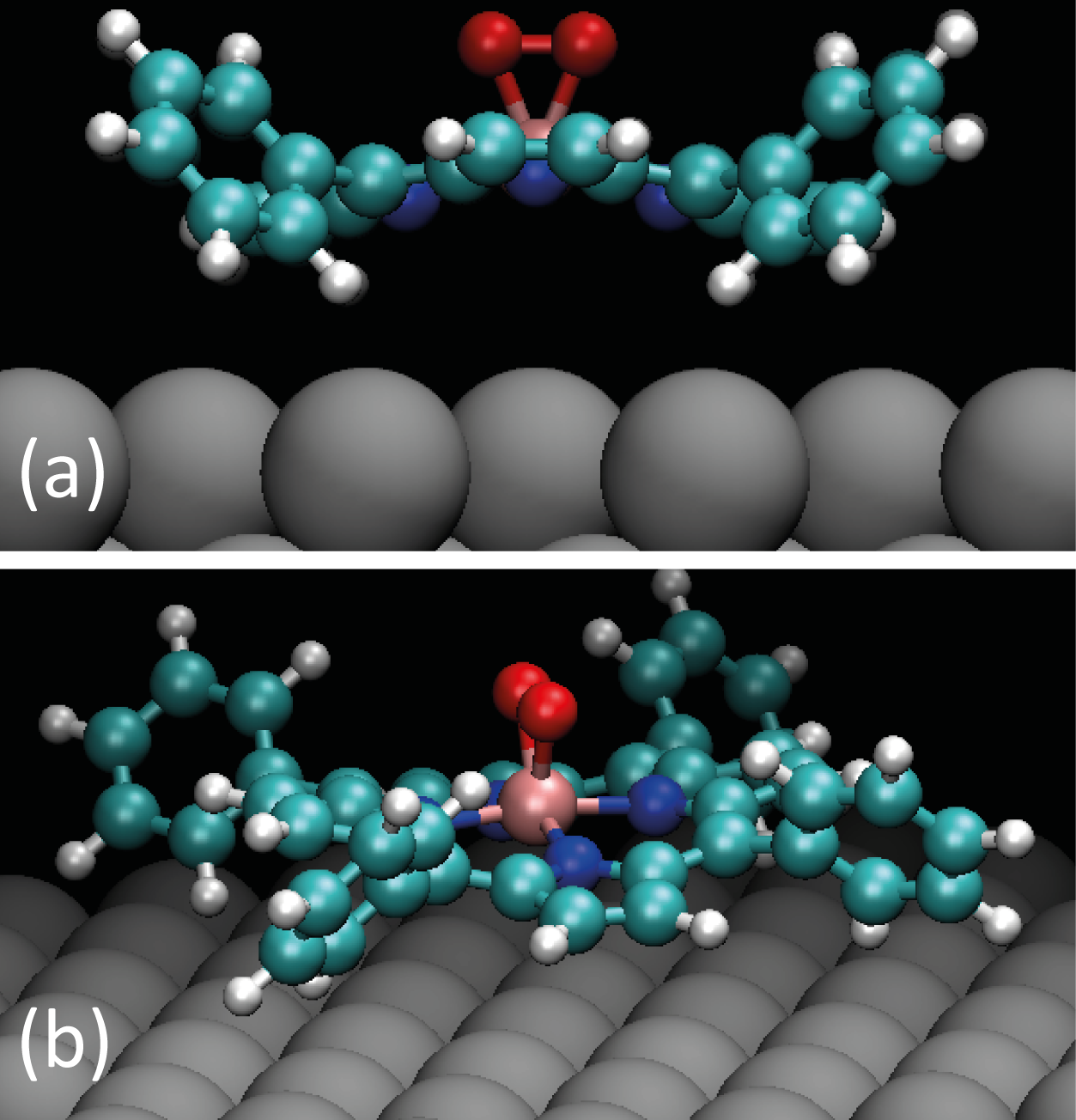
Figure 5.12a shows the side view of the molecule, showing the saddling of the molecule, similar to that of MnClTPP and MnTPP, although the view is rotated by 90° to Figure 5.10 in order to show the orientation of the O–O bond. Initially before relaxation, the bidentate peroxide ligand was positioned “diagonally” across the molecule, with the bond pointing along the line between two phenyl rings. It was first assumed that the O–O ligand would be repelled by the N atoms of the pyrrole rings, however when it was allowed to relax, the O–O bond rotated to lie along the convex slope of the saddle. Several starting positions were attempted, however the O–O bond consistently aligned itself parallel to the Mn–N bonds of the porphyrin, although the alignment along the convex slope (not shown) gave a higher total energy, and so it is assumed that the arrangement shown is the optimal structure.
It is unclear whether this relaxation is due to the O22− anion avoiding the phenyl rings’ steric hindrance [229], or due to an interaction between its electron lone-pairs and the π-electron system of the macrocycle, however hydrogen bonding between the anion and the phenyl rings can be ruled out, due to the former’s preference to orient away from the latter, and the unaltered position of the phenyl rings after relaxation.
It was found that annealing the MnO2TPP monolayer up to 445 K caused the desorption of the peroxide bound to the molecules without disturbing the structure of the monolayer, and STM images of Mn(III)TPP identical to those presented in the previous section were obtained (not shown).
In order to measure the activation energy for the desorption of O2 from the molecules, XPS was performed while the monolayer was annealed, similar to the case of the Cl-ligand desorption. The O 1s spectra are presented in Figure 5.13a, with the temperature at which each spectrum was recorded shown at the right side. The same heating rate as for Cl-desorption of 0.15 K/sec was used.
The XPS data was treated similarly to the previous section describing the Cl desorption, and Figure 5.13b shows the Arrhenius plot for oxygen desorption.
In this case, the activation energy for the desorption of O2 was found to be ΔEO2 = 0.26 ± 0.03eV. This value is lower than that for Cl desorption, indicating a weaker binding for the peroxide ligand than for the Cl ligand.
Interestingly it was noted that it was not possible to directly substitute O2 for Cl at room temperature by exposing the MnClTPP monolayer to O2 gas. This follows intuitively from the fact that the Cl ligand is more strongly bound to the Mn ion than the peroxide ligand, and so it is not energetically favourable for O2 to displace the Cl ligand.