In nature, the cytochrome P450 (CYP) superfamily is a diverse group of enzymes for the oxidation of organic substances. CYPs are proteins based around a heme centre – an Fe(III)-containing porphyrin – and are utilised in many detoxification and biosynthesis pathways [213]. Learning from nature, many biomimetic systems containing Fe(III) or Mn(III) have been studied [214], and have been adapted for use in oxidation chemical reactions [210].
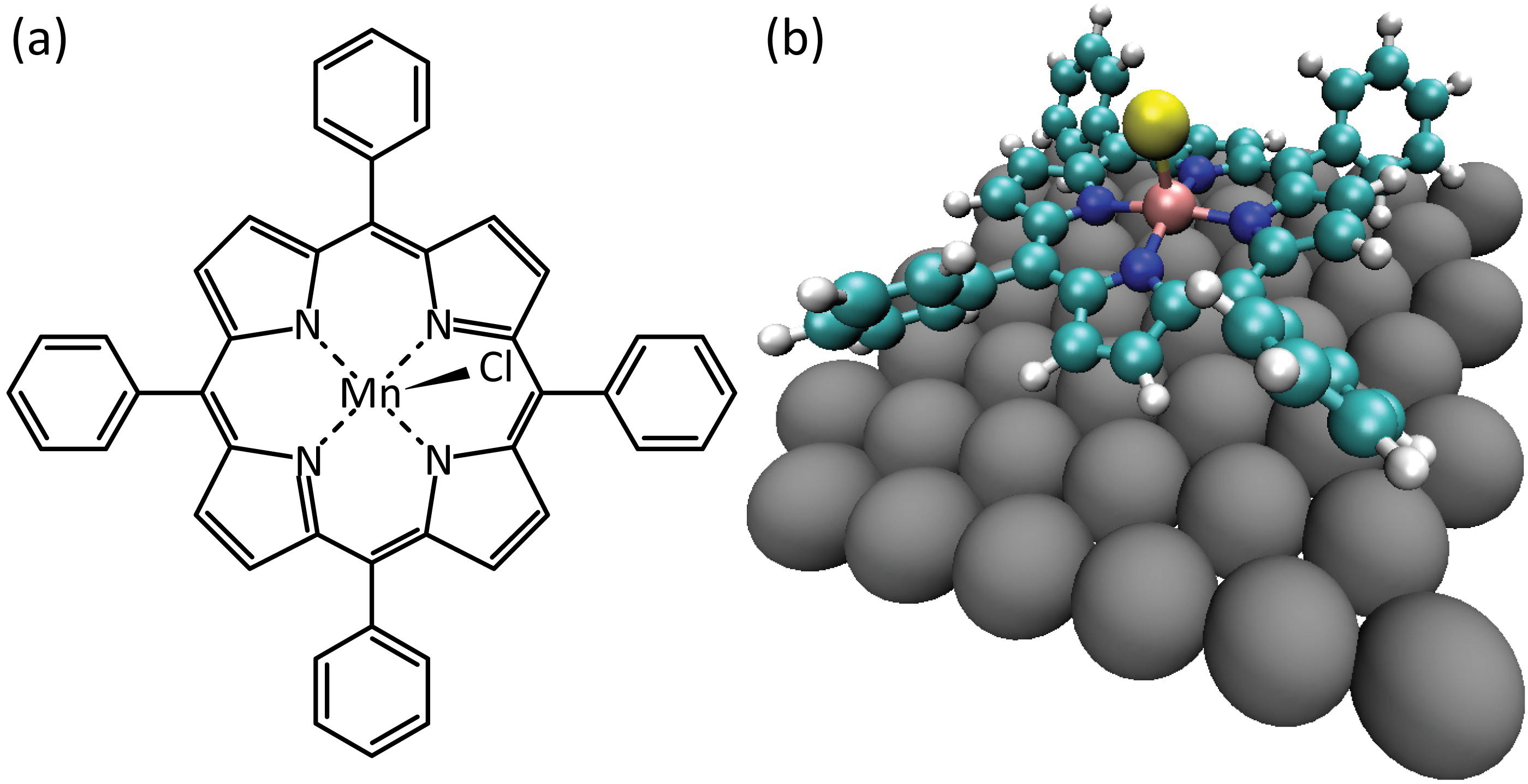
The chemical structure of the porphyrin (5,10,15,20-tetraphenylporphyrinato)Mn(III)Cl is shown in Figure 5.1a. It consists of a porphyrin macrocycle with a Mn core and four phenyl rings attached at the 5, 10, 15 and 20 meso-positions. The Mn ion is in the (III) oxidation state, and is bonded to a Cl ligand pointing out of the plane of the molecule [195]. Also shown in Figure 5.1b is the 3-dimensional model of the molecule on the Ag(111) surface. The molecule’s conformation has been relaxed using DFT calculations and the final optimised atomic positions are shown.

Hulsken et al. have for the first time observed the real-time catalysis of an oxidation reaction by a related, MnCl-centred porphyrin (designated Mn1) using liquid-cell STM [211]. The molecule used was very similar to that in Figure 5.1a, however the phenyl rings at the meso-positions are replaced by long, greasy C11H23 chains to increase its solubility, as shown in Figure 5.2a.
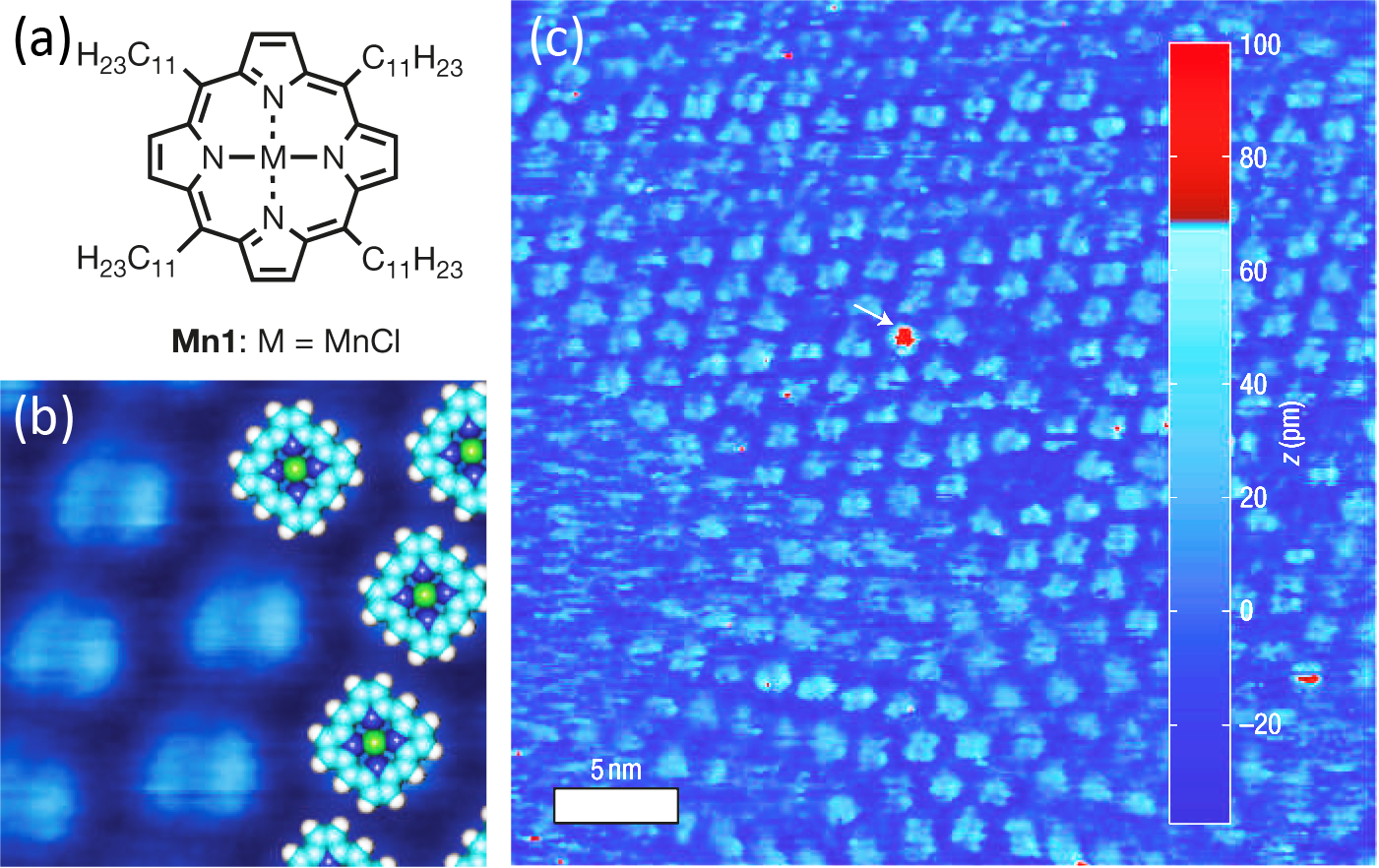
The Mn1 molecules adsorb onto the Au(111) surface in regular arrays, and most molecules exhibit a uniform height. However, in Figure 5.2c, a single molecule shows a larger apparent height. This was attributed to some residual O2 contaminant in the solvent oxidising the Mn1 molecule. The authors note that due to the planar orientation on the surface, and the fact that the free-base porphyrin derivative of Mn1 does not adsorb at the same interface, it is likely that a Au atom of the surface is coordinated to the Mn centre [215].
Similarly, it was shown that O2 did not react with the Mn1 compound in solution without the presence of the surface, indicating that the Au acts as an electron-donating ligand [214]. In order to study the oxidation of the Mn1 monolayer, the authors purged the Ar atmosphere from their system by backfilling with O2 gas, and a sharp increase in the “bright” molecules was observed, implying that these correspond to oxidised Mn1 complexes, as shown in Figure 5.3a.
The mechanism proposed consists of the surface Au atom coordinating to the Mn centre as an axial ligand and causing the Cl ligand to dissociate as a Cl radical, thus reducing the metal centre from Mn(III) to Mn(II). This then allows the reduced Mn1 to bind to an O2 molecule and split it between its own metal centre and that of its neighbour, forming two Mn1=O complexes.
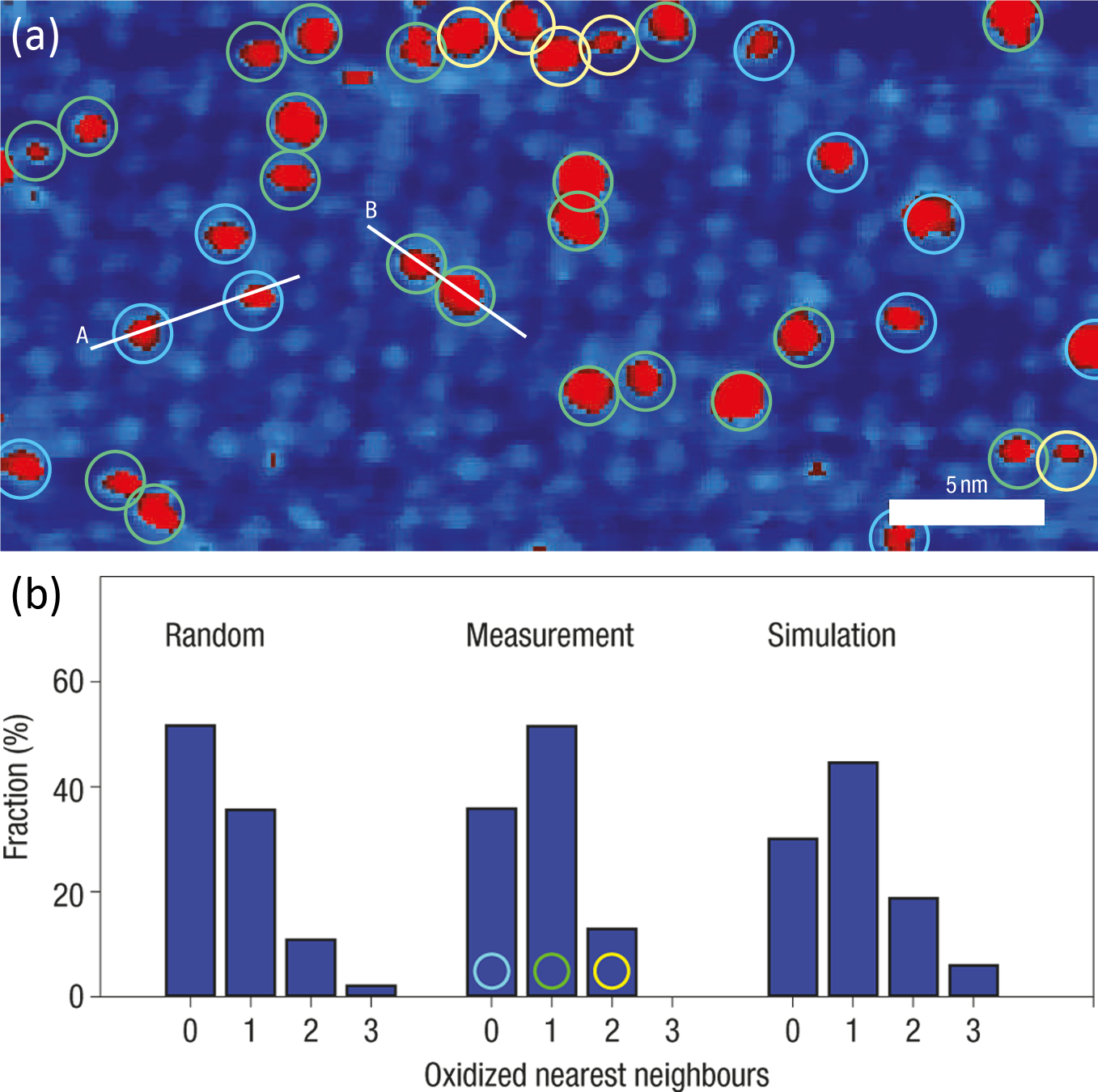
In order to test whether the oxidation of the Mn1 monolayer was random (i.e. each oxidation event is independent of its neighbouring molecules) or whether one O2 molecule was capable of oxidising two Mn1 molecules, simulations were performed and the number of oxidised neighbours of each oxidised molecule was counted. The results are shown in Figure 5.3b, and it is clear that the distribution of oxidised molecules is not random, and instead a pairwise oxidation is favoured.
The final result of this work was the observation of an epoxidation reaction catalysed by the oxidised Mn1 layer, where a sharp decrease in the number of oxidised molecules accompanied the introduction of a precursor molecule, and subsequent analysis of the solution showed the expected epoxide reaction product. Although this seminal paper was conducted in solution, many concepts are common to the present chapter, such as the dissociation of the Cl ligand from MnClTPP, the axial coordination of the dissociated Mn(III)TPP complex by the Ag(111) surface and the subsequent oxidation of the molecules. These results will be elucidated over the following pages.