Auger electron spectroscopy (AES) is a surface-sensitive and most importantly material-sensitive technique based on the Auger effect, which allows the characterisation of a surface’s cleanliness and make-up. The Auger effect was discovered independently by Lise Meitner [51] and Pierre Auger [52] in the 1920s, however it wasn’t until the 1950s that its value as an experimental technique was realised.
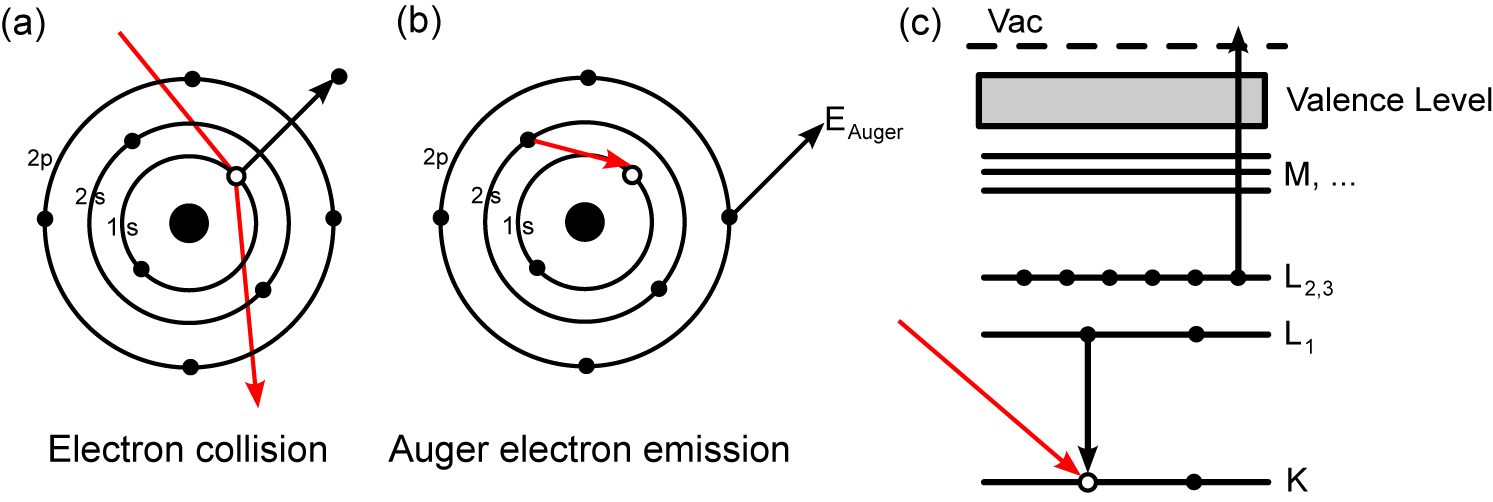
The Auger effect describes the non-radiative emission of an electron after a core vacancy is filled. A schematic of the Auger process is shown in Figure 2.1.

A high-energy electron (or photon) collides with an atom, causing the emission of a core electron (Figure 2.1a). The vacancy left behind is then filled by an outer-shell electron (Figure 2.1b). The energy released then stimulates the emission of an Auger electron, which can be detected and analysed. The kinetic energy of the Auger electron is the difference between the initial transition (L1 → K in Figure 2.1c) and the Auger electron’s ionisation energy (L2,3). The Auger electron is defined by the spectroscopic notation of the energy levels involved in its emission (KL1L2,3 in Figure 2.1).
The K, L, M, etc. nomenclature used to describe the transitions is something of a holdover from the early days of X-ray research. The Nobel laureate Charles G. Barkla first noticed that some X-rays were able to penetrate thicker sheets of metal than others. He began to characterise these differing penetration “strengths” as “A” for more penetrating and “B” for less penetrating, however he worried that further types of X-rays would be discovered, and so reclassified A as K and B as L, to leave the labels A–J free. We now know that his K-type rays were produced by the refilling of the innermost 1s shell in a manner similar to that in Figure 2.1, and there can be no A–J transitions to closer-bound orbitals, however the nomenclature has been kept for historical reasons.
In this way, this terminology is commonly used in spectroscopic techniques
such as AES. An electronic orbital’s principal quantum number n is indicated
alphabetically by its letter, i.e. K (n = 1; 1s), L (n = 2; 2s, 2p), etc. The subscript
on L and higher gives a unique designation to each of the different orbitals which
may have the same n, but different l, s or j quantum numbers, i.e. L1 has
quantum numbers n = 2 and l = 0, and so corresponds to the 2s orbital; L2 is
2p(1∕2) with n = 2, l = 1, j = 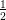 and L3 is 2p(3∕2) with n = 2, l = 1, j =
and L3 is 2p(3∕2) with n = 2, l = 1, j = 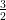 , and so
on in order of increasing energy. When a transition involves multiple orbitals it is
described by the orbitals’ spectroscopic notation, such as the KL1L2,3 transition
in Figure 2.1.
, and so
on in order of increasing energy. When a transition involves multiple orbitals it is
described by the orbitals’ spectroscopic notation, such as the KL1L2,3 transition
in Figure 2.1.
The energies of Auger transitions are specific to each element and its chemical environment, so that its Auger spectrum acts as an elemental “fingerprint”, which can be compared to a catalogue of known spectra. Each element typically has one or more “characteristic peaks” which can be used to detect its presence in complex spectra, e.g. when organic molecules are deposited on a surface, or when testing a surface for possible contamination.
Due to the high linear background signal, the Auger spectrum is usually
represented as a differentiated spectrum, i.e. 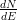 vs. E. Due to the fact that the
differential of a peak (i.e. its slope) will be represented as first a negative and
then positive double peak, it is usual to refer only to the negative peak’s
position.
vs. E. Due to the fact that the
differential of a peak (i.e. its slope) will be represented as first a negative and
then positive double peak, it is usual to refer only to the negative peak’s
position.
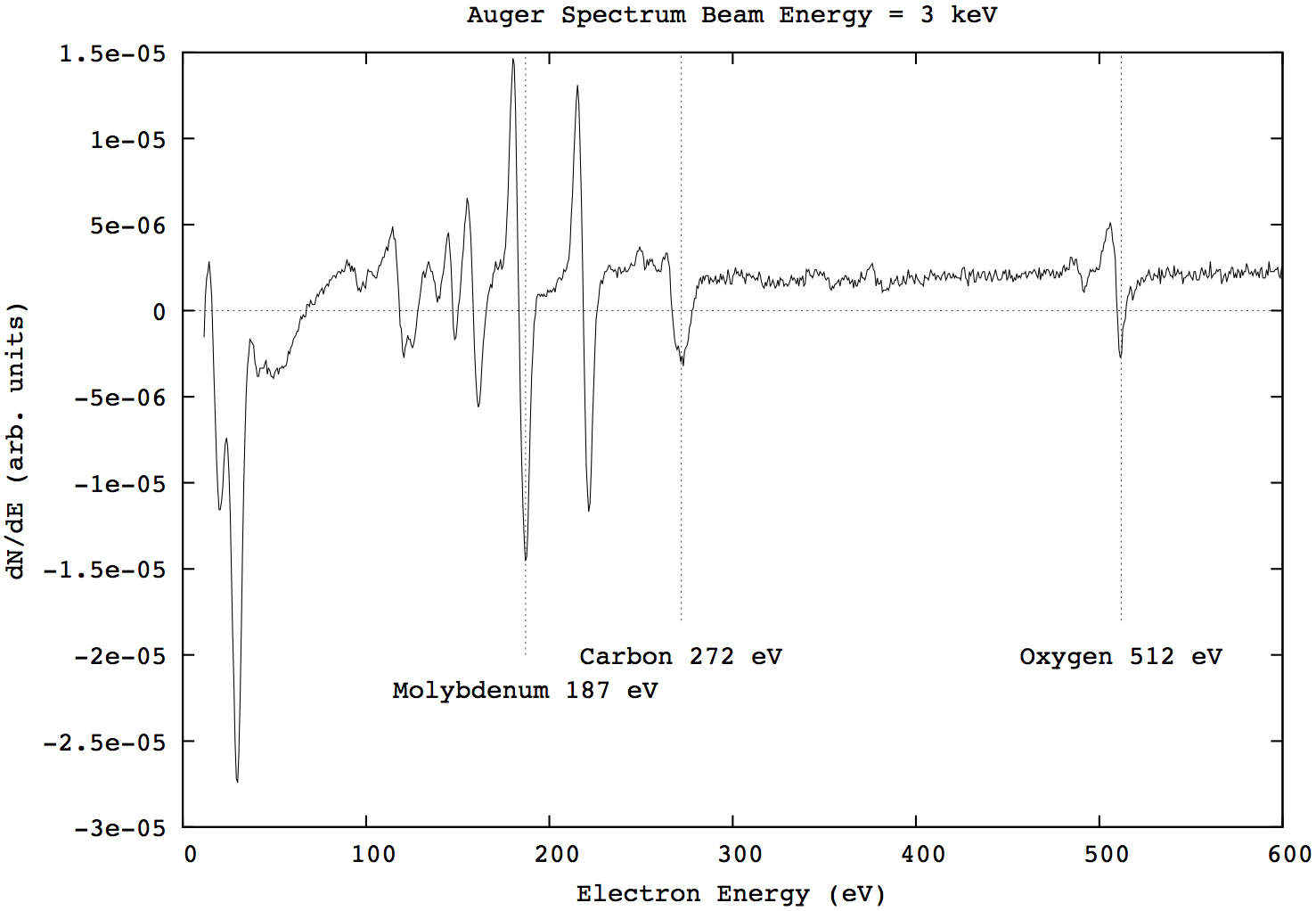
An example spectrum of a dirty Mo(110) sample is shown in Figure 2.2. The characteristic peaks of molybdenum (187 eV), and the two main contaminants, oxygen (512 eV) and carbon (272 eV), are highlighted.
Figure 2.3 shows the experimental set-up of the Auger electron spectroscopy apparatus. 3–5 keV electrons are emitted by the electron gun and collide with the sample. The resulting Auger electrons are passed through a cylindrical mirror analyser (CMA).
The CMA consists of two concentric cylinders, the outer, which can have a bias of -10 V to -2000 V placed upon it, and the inner, which is grounded. Two apertures allow a narrow beam of Auger electrons to enter the space between the two cylinders, where they are repelled by the outer bias. The shape of the CMA is designed so that only those electrons with the same energy as the outer bias can pass through both apertures and arrive at the detector.
In this way the CMA selects the electrons according to their energy by sweeping through energies from 10–2000 eV, however, partial sweeps can also be used to more quickly screen samples, and to limit the time they are subjected to high energy 3–5 keV electrons, which can damage surface structures.