Understanding the mechanisms that control matter on the smallest level is one of the key goals of materials science. When deposited onto a surface, atoms and molecules arrange themselves into novel structures, often resulting in emergent behaviour and interesting phenomena.
This self-assembly is a fundamental topic of surface science and nanotechnology [1–20] and requires a broad understanding of the complex interplay between the adsorbates and the surfaces which support them. Organic molecules represent an interesting avenue for the study of self-assembly due to the myriad different species and activities available.
Three organic molecules will be examined in this thesis: the fullerene C60; and two porphyrin derivatives, (5,15-diphenylporphyrinato)Ni(II), designated NiDPP for convenience, and (5,10,15,20-tetraphenylporphyrinato)Mn(III)Cl, MnClTPP.
Fullerenes are a class of carbon allotropes consisting of sp2-hybridised carbon atoms arranged in three-dimensional, closed-cage structures. The fullerene family encompasses such exotic materials as the single atomic sheets of graphene, pseudo-one-dimensional carbon nanotubes and spherical C60 buckminsterfullerenes, from which the family derives its name. It is the last of these, C60, which will be described in detail in this thesis.
Many potential applications of C60 fullerenes have been postulated, and they are the basis of active areas of research. When added to bulk heterojunction solar cells as electron acceptors, in some cases they have been shown to increase efficiencies immensely [21], indicating that C60 may be very important for the future development of organic solar cells. C60 has also shown some promise as a future data storage medium [22], with precise, repeatable non-volatile storage possible with a data density approaching the theoretical limit of a single-molecular bit size.
However, even though many applications for C60 have been theorised since their discovery in 1985 [23], many open fundamental questions remain. When deposited onto metallic surfaces at room temperature, C60 molecules generally form close-packed islands, and start to nucleate at substrate step edges.
Thin C60 films on metal [18, 24–37] and semiconductor [22, 38–42] surfaces have been widely studied, however reports of their self-assembly on oxides are much more rare [43–45]. It is for this reason that the interaction between C60 and the oxide nanorows of WO2/W(110) is examined in this work.
Due to their spherical symmetry, C60 molecules have enough energy to freely rotate at room temperature. However during cooling, molecule–substrate interactions overcome the molecules’ kinetic energy, resulting in two separate phase transitions. A number of different effects are observed, such as spinning perpendicular to the surface, molecular charging and the eventual freezing in place of the C60 molecules’ orientations.
The interaction between the molecules and substrate is dependent on the metal surface and can result in adsorbate-induced surface reconstruction, which can be accompanied by charge transfer between the surface and the molecules [25], however, in this thesis, no induced surface reconstructions are observed on the strained-commensurate WO2/W(110) surface [46–48].

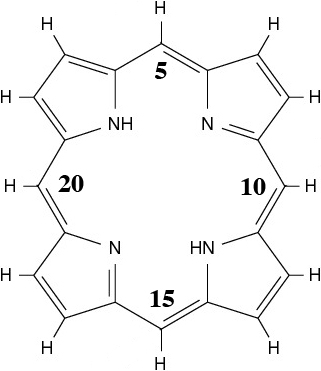
Porphyrins are another family of organic molecules. The simplest form, porphine, consists of an unsaturated macrocyclic carbon structure made up of four pyrrole rings and four C atoms in the meso positions, with two of the pyrrole rings’ nitrogen atoms each bonded to a delocalised hydrogen atom, as shown in Figure 1.1. This simple structure forms the basis for many of the most important chemicals both in industry and the natural world. If the central hydrogen atoms are removed, the four nitrogen atoms can act as ligands for a transition metal ion. When an Fe(II) ion is added to the porphyrin, it becomes a heme, the metal complex which is the key component in the oxygen-storage capacity of red blood cells (haemoglobin), vital for most animal life on Earth. When Mg(II) is substituted instead of Fe(II), the resulting structure forms the basis for chlorophyll, without which plant life on Earth would be unable to produce food from the sun. Porphyrins can form complexes with many transition metal ions, and those of Fe, Ni and Co have been shown to exhibit magnetic properties [49].
As well as by transition metal binding, complex porphyrins can be formed through the attachment of side chains to the outer carbon ring, most commonly at the 5, 10, 15 and 20 positions (as indicated in Figure 1.1). Such substituent groups alter both the bonding of the molecule and its electronic structure.
Depending on the central transition metal’s oxidation state, it can bond to a fifth ligand pointing perpendicularly out of the plane of the porphyrin macrocycle, as in the case of MnClTPP. This ligand can be physically manipulated by annealing the organic layer or chemically altered by subjecting the molecules to reactive gas. The nature of the ligand affects the electronic structure of the porphyrin, which can be probed by scanning tunneling microscopy and X-ray spectroscopy.
In addition to the organic molecule examined, the choice of surface is crucial, with some substrates acting as templates for the the molecules’ self-organisation.
When W(110) is oxidised it forms an ultra-thin O–W–O trilayer on its surface. This trilayer corresponds to the WO2(010) structure, however due to the slight lattice mismatch between W(110) and WO2(010), the surface reconstructs to form a strained-commensurate overlayer exhibiting ordered oxide nanorows oriented along the [37] and [3] directions of the substrate.
Such nanorows can be used to template the deposition of C60 molecules, and the morphology and chemistry of the surface cause the molecular layer to exhibit interesting physical and chemical properties.
In order to highlight the effect of the molecule-substrate interaction,
NiDPP is deposited onto two different, but related, surfaces, Ag(111) and
Ag/Si(111)-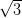 ×
×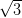 R30°. Ag(111), which will also act as the substrate for
MnClTPP deposition, is a simple close-packed noble metal surface, while
Ag/Si(111) has the so-called honeycomb-chain-trimer structure, consisting of
pseudo-hexagons of Ag surrounding Si trimers. This more complex and reactive
surface interacts more strongly with the NiDPP molecules, affecting their
self-assembly [50].
R30°. Ag(111), which will also act as the substrate for
MnClTPP deposition, is a simple close-packed noble metal surface, while
Ag/Si(111) has the so-called honeycomb-chain-trimer structure, consisting of
pseudo-hexagons of Ag surrounding Si trimers. This more complex and reactive
surface interacts more strongly with the NiDPP molecules, affecting their
self-assembly [50].
In chapter 3, C60 molecules are deposited onto the WO2/W(110) surface, which exhibits a regular array of oxide nanorows. This causes some molecules to lie topologically lower than others, and they appear as dark chains in STM images. The ultrathin WO2 layer also decouples the C60 molecules from the underlying W(110) substrate, allowing charge to accumulate on individual C60 molecules.
Chapter 4 discusses the effect two different, but related, surfaces have on the
self-assembly of NiDPP molecules. Ag(111) is a relatively-simple, inert surface
which leads to a close-packed structure, whereas the complex Ag/Si(111)-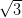 ×
×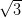 R30° surface is more reactive and gives rise to a random domain structure within
the NiDPP thin film.
R30° surface is more reactive and gives rise to a random domain structure within
the NiDPP thin film.
Finally, MnClTPP is deposited onto the Ag(111) surface in chapter 5, again leading to a close-packed structure. However, this unreactive surface still plays a vital role in the transformation of the axial Cl-ligand. When the Cl is removed from the molecule by annealing, an atom from the Ag(111) surface acts as a fifth ligand, stabilising the Mn(III)TPP state.