Summary
In this thesis, three organic molecules are examined on three distinct surfaces by a
variety of surface science techniques, foremost among them being scanning
tunneling microscopy (STM).
When deposited on the WO2/W(110) surface, C60 fullerenes self-assemble into
islands which grow from the substrate’s inner step edges, forming a close-packed
monolayer.
At some bias voltages, chains of molecules sit topologically lower in height
than others, because they lie in the grooves between the WO2 nanorows. When
the sample is imaged at room temperature, all C60 are spinning too fast for the
STM to resolve, and so appear as featureless spheres. However, when cooled to
78 K, the molecules’ motion is frozen and their inner orbital structure can be
seen.
Depending on the rate of cooling, an orbital-ordered monolayer (slow cooling),
or a glassy monolayer with random molecular orientations (fast quenching) can be
achieved.
During cooling, two phase transitions are observed. As the temperature is
decreased, a structural transition occurs at 259 K when some molecules begin to
freeze, and a kinetic transition is observed at 220 K when all molecular motion has
stopped.
Between the two transitions, several dynamic behaviours are observed. Some
molecules which spin like a top appear as a ring with either a dark centre or
a protrusion. Others change their apparent height over time, which is
attributed to them gaining and losing an electron, and thus changing their
density of states and hence their conductance, as measured by STM. This
charging and discharging is accompanied by the molecules’ rotation on the
surface, as they interact with their neighbours and the underlying oxide
substrate. The observations obtained by STM are supported by DFT
calculations.
The second molecule examined was nickel diphenyl-porphyrin (NiDPP)
deposited on the Ag(111) and Ag/Si(111)-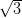 ×
×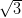 R30° surfaces. On the inert
Ag(111) surface, NiDPP self-assembles into a single close-packed domain, as
shown by STM and low energy electron diffraction (LEED). The molecules
exhibit a tilted-row structure with a slightly oblique unit cell, with one
diagonal of the unit cell aligned with the step edges of the underlying
substrate.
R30° surfaces. On the inert
Ag(111) surface, NiDPP self-assembles into a single close-packed domain, as
shown by STM and low energy electron diffraction (LEED). The molecules
exhibit a tilted-row structure with a slightly oblique unit cell, with one
diagonal of the unit cell aligned with the step edges of the underlying
substrate.
In contrast, on the Ag/Si(111)-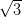 ×
×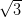 R30° surface the NiDPP molecules
adopt one of three equivalent orientations, dictated by the surface symmetry. The
molecules pack closer together, and this strain is accommodated by a stronger
interaction with the more-reactive surface. Three domains rotated by 120° to one
another are observed to be randomly distributed over the surface due to
nucleation from molecules adopting one of the three orientations upon contact
with the surface.
R30° surface the NiDPP molecules
adopt one of three equivalent orientations, dictated by the surface symmetry. The
molecules pack closer together, and this strain is accommodated by a stronger
interaction with the more-reactive surface. Three domains rotated by 120° to one
another are observed to be randomly distributed over the surface due to
nucleation from molecules adopting one of the three orientations upon contact
with the surface.
Finally, another porphyrin, manganese-chloride tetraphenyl-porphyrin, or
MnClTPP, has also been studied on the Ag(111) surface. Upon deposition, the
molecule adopts a saddle conformation and the axial chloride ligand points out
into the vacuum. The monolayer assembles into the typical square close-packed
geometry commonly observed for tetraphenyl-porphyrins.
When it is annealed up to 510 K, the Cl-ligand is removed, but the Mn(III)
oxidation state is stabilised through interaction with the substrate, as observed by
X-ray absorption spectroscopy (XAS).
Exposure to molecular oxygen oxidises the central Mn ion to the Mn(IV)
state and the oxygen molecule binds to the centre as a bidentate peroxide
ligand. This MnO2TPP state is stable up to 445 K, whereupon the O2 is
lost from the molecule, and the Mn ion is reduced back to the Mn(III)
state. From core-level X-ray photoelectron spectra (XPS) taken from
the Cl 2p and O 1s levels, the activation energies for Cl and O2 removal
were found to be ΔECl = 0.35 ± 0.02eV and ΔEO2 = 0.26 ± 0.03eV,
respectively.
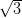 ×
×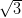 R30° surfaces. On the inert
Ag(111) surface, NiDPP self-assembles into a single close-packed domain, as
shown by STM and low energy electron diffraction (LEED). The molecules
exhibit a tilted-row structure with a slightly oblique unit cell, with one
diagonal of the unit cell aligned with the step edges of the underlying
substrate.
R30° surfaces. On the inert
Ag(111) surface, NiDPP self-assembles into a single close-packed domain, as
shown by STM and low energy electron diffraction (LEED). The molecules
exhibit a tilted-row structure with a slightly oblique unit cell, with one
diagonal of the unit cell aligned with the step edges of the underlying
substrate.
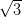
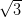 R30
R30